Vapor-liquid equilibrium
The vapor-liquid equilibrium (mostly referred to as VLE after English Vapor-Liquid Equilibrium ) is a phase equilibrium in which a liquid and a vapor or gas are in thermodynamic equilibrium . The vapor-liquid balance is also characterized by the fact that the amount of substance that evaporates corresponds to the amount of substance that condenses. In addition, the chemical potential is the same in both phases.
Basics
A vapor-liquid equilibrium is clearly determined for pure substances by the parameters pressure and temperature ; for mixtures, the compositions of the liquid and vapor phases must be specified. Compositions are mostly as a mole fraction indicated (mole fraction) x for the liquid phase and y for the vapor phase. A vapor-liquid equilibrium exists in the area between the triple and the critical point . The pressure that is established or is established in the vapor-liquid equilibrium is called the saturation vapor pressure .
Mixtures
Mixture-vapor-liquid equilibria are characterized by the fact that the composition of the liquid and vapor phases usually differ. This effect arises from the different volatility and thus the different partial pressure of the substances involved and is used in separation processes , especially rectification , to separate mixtures. The low boiler accumulates in the vapor phase, the high boiler, however, in the liquid phase. Rectification is currently the most frequently used process for material separation, for example in petroleum refineries . Systems in which the composition does not differ are called azeotropic and cannot be separated by evaporation.
Parameters
A number of parameters have been defined to describe the vapor-liquid equilibrium of a mixture:
- Separation factor that describes the ratio of the saturation vapor pressures of the components involved
- K factor that describes the ratio of the substance proportions of a component in vapor and liquid
- Relative volatility , which describes the relationship between two K-factors
These key figures all serve to be able to recognize in a simple way whether a rectification should be carried out in a meaningful way.
Typical representations
Pure substance equilibria are mostly shown simply as temperature-pressure diagrams, but the logarithm of the pressure is often plotted against the reciprocal value of the temperature, as this representation results in an approximately straight line.
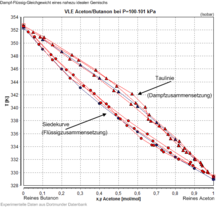
Mixture-vapor-liquid equilibria are mostly determined experimentally at constant temperature or constant pressure. Therefore, VLE of binary mixtures are usually represented at constant pressure as temperature versus composition and at constant temperature as pressure versus composition in the liquid and vapor phases. The concentration data in the steam are referred to as the dew line, while the sequence of the liquid compositions is known as the boiling line. An alternative commonly used representation is the plot of the two compositions in liquid and vapor x against y.
Modeling
A pure substance-vapor-liquid equilibrium can be described by simple equations , often derived from the Clausius-Clapeyron equation , such as the Antoine equation . These equations use substance-specific parameters that have been fitted to experimental vapor pressure data.
A mixture-vapor-liquid equilibrium can be described as a first approximation by Raoult's law . However, this requires ideal behavior of the substances involved. To take real behavior into account, activity coefficient models such as the Non-Random-Two-Liquid-Modell (NRTL) or UNIQUAC (Universal Quasichemical) are mostly used , which describe the excess energy of Gibb. The Unifac method is suitable for estimating the activity coefficients.
literature
- DECHEMA Chemistry Data Series, Volume 1, Vapor-Liquid Equilibrium Data Collection, Various authors.
- " The Properties of Gases & Liquids ", Bruce E. Poling, John M. Prausnitz, John P. O'Connell, McGraw-Hill Professional, 2000, ISBN 0-07-011682-2
- Perry, RH and Green, DW "Perry's Chemical Engineers' Handbook", 7th edition, McGraw-Hill, 1997, ISBN 0-07-049841-5
See also
- Henry Law
- Dortmund database (collection of vapor-liquid equilibrium data of mixtures and saturation vapor pressures of pure substances, basis of the DECHEMA Chemistry Data Series)