Zeotropic mixture
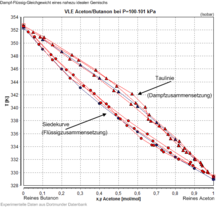
A mixture of chemical substances is called zeotropic if the composition of liquid and vapor in the vapor-liquid equilibrium is always different. This means that the dew curve and boiling curve do not touch at any point. Mixtures whose dew and boiling curves touch at least one point and thus the composition in vapor and liquid is the same are called azeotropic mixtures.
The vapor pressure and boiling temperature of a zeotropic mixture are characterized by the fact that they are always between the vapor pressures and boiling temperatures of the pure substances, while in azeotropic mixtures a pressure maximum / temperature minimum or pressure minimum / temperature maximum occurs that is outside the range limited by the pure substance values.
meaning
Zeotropic mixtures can be separated by distillation or rectification , i.e. repeated evaporation and condensation, in separation columns. Their separation factors are never 1, but can be so low that separation by rectification is technically impossible.
From a technical point of view, zeotropy and azeotropy are also of greater importance in the case of refrigerant mixtures, where a distinction is made between zeotropic (R4xx) and azeotropic mixtures (R5xx) when the name is given. Zeotropic mixtures have the disadvantage that their composition changes during evaporation and condensation. This also changes the boiling points and other properties, especially the caloric variables that are important in cooling technology, such as enthalpy of vaporization and heat capacity . Azeotropic mixtures, on the other hand, behave like pure substances with constant properties in the azeotropic composition. In the case of zeotropic mixtures, care must therefore be taken to ensure that the differences remain small and therefore zeotropic mixtures with small separation factors are preferred.
Occurrence
Zeotropy occurs when the components involved in a mixture are chemically similar or their boiling point difference is very large. For example, mixtures that only consist of alkanes or monofunctional ketones are chemically similar . On the other hand, azeotropy occurs with smaller boiling point differences and with substances with different functional groups, such as a mixture of methanol and chloroform .
A mixture can be both zeotropic and azeotropic, since the azeotropic composition is temperature and pressure dependent, sometimes stronger and sometimes weaker. For example, the mixture of ethanol and water behaves zeotropically below approximately T = 305 K and p = 12 kPa, while azeotropy occurs at higher temperatures and pressures. At normal pressure, the composition is about 90 mol% ethanol and 10 mol% water. At the limit, however, the differences between the compositions of the vapor phase and the liquid phase as well as the relative volatility are still very small, so that a technical use of the effect near the azeotrope / zeotrope transition is hardly possible, but only at significantly lower temperatures and To press.
description
Zeotropy is the qualitative statement about a mixture at a given pressure and temperature, that the liquid and the vapor always have different compositions. An indication of a special composition is therefore not useful.