4-chlorobutanol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-chlorobutanol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 9 OCl | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 107.56 g mol −1 | ||||||||||||||||||
| density |
1.090 g cm −3 (20 ° C) |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Refractive index |
1.4518 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
4-chlorobutanol is an organic chemical compound . It is a 4-position chlorinated n- butanol and thus belongs to the group of chlorobutanols .
General
4-Chlorobutanol can be obtained by introducing hydrogen chloride into tetrahydrofuran and is an intermediate product in the production of 1,4-dichlorobutane .
It can also be made by the reaction of 1,4-butanediol with thionyl chloride in the presence of pyridine .
Reactions
4-Chlorobutanol reacts with hydrogen chloride to form 1,4-dichlorobutane with elimination of water.
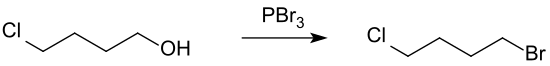
When reacting with phosphorus tribromide , 1-bromo-4-chlorobutane is formed.
When 4-chlorobutanol is heated to above 85 ° C., hydrogen chloride is split off and tetrahydrofuran is formed.
safety instructions
4-chlorobutanol was found to be moderately toxic in mice with oral LD 50 values of 990 mg / kg and> 100 mg / kg after intraperitoneal administration. Changes in the liver, muscle twitching and gastric bleeding occurred in the animals at high doses.
Individual evidence
- ↑ a b Data sheet 4-chloro-1-butanol (PDF) from Merck , accessed on March 13, 2012.
- ↑ Data sheet 4-chloro-1-butanol from AlfaAesar, accessed on March 13, 2012 ( PDF )(JavaScript required) .
- ^ A b W. R. Kirner, G. Holmes Richter: Tetramethylene Glycol and Tetramethylene Chlorohydrin . In: Journal of the American Chemical Society . 51, No. 8, 1929, pp. 2503-2506. ISSN 0002-7863 . doi : 10.1021 / ja01383a033 .
- ↑ a b Data sheet 4-chloro-1-butanol, technical grade, ~ 85% from Sigma-Aldrich , accessed on March 13, 2012 ( PDF ).
- ^ Journal of Organic Chemistry . Vol. 21, Pg. 739, 1956.
- ↑ a b Entry on 4-chlorobutanol in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Journal for the entire hygiene and its border areas . Vol. 26, Pg. 17, 1980.
- ↑ D. Starr, RM Hixon: Tetramethylene Chlorohydrid In: Organic Syntheses . 17, 1937, p. 84, doi : 10.15227 / orgsyn.017.0084 ; Coll. Vol. 2, 1943, p. 571 ( PDF ).
- ↑ a b Author collective: Organikum . 22nd edition, Wiley-VCH, 2004, ISBN 978-3-527-31148-4 .
- ↑ a b c D. Starr, RM Hixon: Reduction of Furan and the Preparation of Tetramethylene Derivatives in J. Am. Chem. Soc. 1934 , 56 (7), pp. 1595-1596. doi : 10.1021 / ja01322a041