Reactive dyes
Reactive dyes are a group of textile dyes used to dye cotton , wool and polyamide fibers . During the dyeing process, a covalent chemical bond is formed between the dye and the functional groups of these fibers.
Chemical properties
Reactive dyes consist of a coloring chromophore with one or more solubilizing groups and one or more reactive groups, the so-called reactive anchors . Common chromophores are azo dyes , anthraquinone dyes , phthalocyanines , formazans and triphendioxazine dyes . As a rule, sulfonic acid groups are contained as solubilizing groups .
An important reactive anchor system are halogen-substituted aromatic heterocycles , such as, for example, monochlorotriazines , monofluorotriazines , difluoropyrimidines , difluorochloropyrimidines and dichloroquinoxaline carbonamides . The dichlorotriazine group used in the first commercial reactive dyes no longer plays a significant role today.
CHR = chromophore, R = aromatic or aliphatic amine
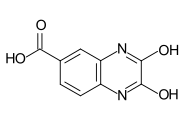
Important intermediate products for the production of these reactive dyes are cyanuric chloride (TCT), cyanuric fluoride (TFT), trichloropyrimidine , trifluoropyrimidine , tetrafluoropyrimidine and trifluorochloropyrimidine , as well as 2,3-dihydroxyquinoxaline-6-carboxylic acid and 2,3-dichloroquinoxaline-6-carboxylic acid chloride .
The fluoroheterocycles are prepared from the corresponding chloroheterocycles by halogen exchange. The dichloroquinoxaline carboxylic acid chloride is obtained by reacting dihydroxyquinoxaline carboxylic acid with thionyl chloride .
Under alkaline dyeing conditions, these reactive dyes react with the hydroxyl groups of the fibers, splitting off hydrogen chloride (HCl) or hydrogen fluoride (HF) and forming a stable covalent ether bond .
Reaction of reactive dyes with heterocyclic, halogen-containing reactive anchors during the dyeing process
The second major reactive anchor system is the so-called vinyl sulfone group in the vinyl sulfone dyes . The vinyl sulfone group reacts during the dyeing process with the nucleophilic groups of the fiber in the sense of a Michael addition . In this case, too, a stable ether bond is formed. In the case of many dyes, the vinyl sulfone group is in a protected form, for example as a sulfuric acid half-ester. The vinyl sulfone group is only formed by the elimination of sulfuric acid under the alkaline dyeing conditions .
Reaction of reactive dyes with vinyl sulfone reactive anchors during the dyeing process
In addition to the mono anchor dyes, which contain only one reactive anchor group, a large number of high-performance bifunctional reactive dyes have been developed over the years, which are characterized by a high rate of fixation during the dyeing process. Important commercial bifunctional reactive dyes contain either two VS groups (homobifunctional dyes) or a combination of VS reactive anchor and heterocyclic fluorine or chlorine reactive anchor (heterobifunctional dyes).
Commercially important reactive dyes
- CI Reactive Black 5 is one of the oldest and, in terms of quantity, the world's largest reactive dye. It is used under various trade names and as a main component in many different black mixtures. It is a navy blue disazo dye with two VS reactive anchors. In combination with red or orange dyes, deep black colors can be obtained.
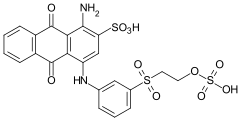
- CI Reactive Blue 19 is a monofunctional vinyl sulfone reactive dye from the class of anthraquinone dyes. It is also a product from the early days of reactive dyes, which is still of great commercial importance. The dyeing on cotton produces a brilliant blue.
- CI Reactive Orange 107 is a simple monofunctional azo dye with an aromatic vinyl sulfone reactive group that is introduced into the molecule via the diazo component, parabase ester .
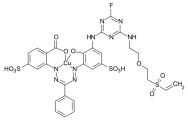
- CI Reactive Red 239 is a heterobifunctional reactive dye with a monochlorotriazine and an aromatic vinyl sulfone reactive anchor.
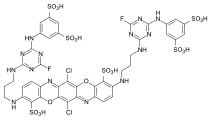
- CI Reactive Blue 235 is a heterobifunctional reactive dye with a monofluorotriazine and an aliphatic vinyl sulfone reactive anchor and thus an example of a relatively new development in the field of reactive dyes. The product's chromophore is a formazan-Cu complex dye .
- CI Reactive Blue 204 is a homobifunctional reactive dye with two monofluorotriazone reactive anchors and belongs to the triphendioxazine dyes.
- CI Reactive Blue 7 is a reactive dye from the class of phthalocyanine dyes .
- CI Reactive Red 1 is a monofunctional reactive dye with a dichlorotriazine reactive anchor
Reactive dye assortments
Examples of commercial reactive dye ranges are
- Drimaren dyes (reactive anchors: difluorochloropyrimidine, difluropyrimidine, as well as combination with vinyl sulfone groups). Drimaren is a protected trade name of Archroma , formerly Clariant , or Sandoz .
- Levafix dyes (reactive anchors: monofluorotriazine, difluropyrimidine, difluorochloropyrimidine, dichloroquinoxaline, as well as combination with vinyl sulfone groups). Levafix is a registered trade name of the DyStar Group, formerly Bayer AG .
- Novacron dyes (reactive anchor: monoflorotriazine, sometimes in combination with monochlorotriazine and VS groups). Novacron is a registered trade name of the Huntsman Corporation , earlier name Cibacron dyes as a trade name of Ciba Specialty Chemicals .
- Procion dyes (reactive anchor: monochlorotriazine). Procion is a registered trade name of the DyStar Group, formerly Imperial Chemical Industries (ICI), or BASF .
- Remazol dyes (reactive anchor: vinyl sulfone groups, sometimes in combination with monochlorotriazine). Remazol is a registered trade name of the DyStar Group, formerly Hoechst AG .
- Sumifix dyes (reactive anchor: vinyl sulfone groups in combination with monochlorotriazine). Sumifix is a registered trade name of Sumitomo .
Reactive dyeing
The dyeing of cotton with reactive dyes takes place under alkaline conditions. The required dyeing temperature essentially depends on the reactive anchor. The relatively inert monochlorotriazine dyes require temperatures> 80 ° C. They are therefore also known as hot dyers. The much more reactive monofluorotriazine dyes, on the other hand, can be used at very low temperatures of <40 ° C. These are so-called cold dyers. Vinyl sulfone dyes can be used over a fairly wide range of temperatures.
During the dyeing process, undesirable side reactions of the dyes with water occur, especially at higher temperatures. The hydrolyzed and consequently unfixed dye components must therefore be washed out again to achieve the desired wet fastness.
history
The development of reactive dyes is based on the discovery made in 1954 by Imperial Chemical Industries (ICI) that certain dyes with a dichlorotriazine residue can give very washfast dyeings under alkaline dyeing conditions. The first reactive dyes with a dichlorotriazine anchor were introduced onto the market by ICI in 1956, followed by the first vinyl sulfone dyes by Hoechst AG in 1957. Important milestones in further development were the introduction of the monofluorotriazine and difluorochloropyrimidine reactive anchors around 1980 (Bayer AG / Ciba-Geigy / Sandoz), as well as the introduction of bifunctional VS / monochlorotriazine dyes by Sumitomo (1981) and bifunctional VS / monofluorotriazine dyes by Ciba-Geigy.
literature
- K. Venkataraman (Ed.): The Chemistry of Synthetic Dyes . Reactive Dyes. tape VI . Academic Press, New York, London 1972 ( limited preview in Google Book search).
- Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 113 ff . ( limited preview in Google Book search).
- Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 225 ff . ( limited preview in Google Book search).
- P. Rys, H. Zollinger: dye chemistry. Verlag Chemie. Weinheim.
- Reactive Dyes: Ullmann's Encyclopedia of Industrial Chemistry, Volume A22, pp. 651ff (1993).
- Norbert Welsch, Claus Chr. Liebmann: Colors: nature, technology, art . 2nd Edition. Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1563-2 ( limited preview in Google book search).
Individual evidence
- ↑ Entry on reactive anchors. In: Römpp Online . Georg Thieme Verlag, accessed on April 4, 2019.
- ↑ Patent DE1019025 : Process for the production of monoazo dyes. Applied November 19, 1955 , published November 7, 1957 , applicant: ICI Ltd., inventor: William Elliot Stephen.
- ↑ Patent DE1062367 : Process for the production of monoazo dyes. Applied on November 19, 1955 , published July 13, 1959 , applicant: ICI Ltd., inventor: William Elliot Stephen.
- ↑ Hans Beyer, Wolfgang Walter: Textbook of organic chemistry . 18th edition. S. Hirzel Verlag, Stuttgart 1978, ISBN 3-7776-0342-2 , p. 521 .
- ^ Heinrich Zollinger: Chemism of the reactive dyes . In: Angewandte Chemie . tape 73 , no. 4 , February 21, 1961, p. 125 , doi : 10.1002 / anie.19610730402 .