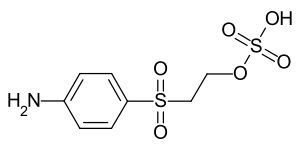
Parabase ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Parabase ester | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 11 NO 6 S 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 281.3 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Parabase ester or 2 - [(4-aminophenyl) sulfonyl] ethyl hydrogen sulfate ( IUPAC ) is an industrial intermediate for the synthesis of organic, water-soluble reactive dyes . The aniline derivative contains the sulfonylethyl hydrogen sulfate substituent, a functional group that splits off sulfuric acid under alkaline dyeing conditions . The reactive vinyl sulfone group (VS group) thus formed forms a covalent bond with the functional groups of the cellulose fibers . Parabase ester is the most important intermediate in terms of volume for reactive dyes with this reaction principle ( vinyl sulfone dyes or VS dyes).
Manufacturing
Starting from aniline , parabase ester ( 6 ) is accessible in a five-step synthesis:
To protect the amino group , aniline ( 1 ) is first converted into acetanilide ( 2 ) with acetic anhydride . Subsequently, by chlorosulfonation with chlorosulfonic acid and thionyl chloride introduced Chlorsufonylgruppe ( 3 ). The p -acetanilide sulfinate ( 4 ) is obtained by reduction with sodium sulfite . In the next step, the sulfinate is converted into 2 - [(4-aminophenyl) sulfonyl] ethanol ( 5 ) ( N-acetyl-parabase ) by ethoxylation . It is then esterified with sulfuric acid , the acetyl protective group being split off at the same time.
use
Parabase ester is used as a diazo component in the manufacture of various azo dyes . In the case of the world's largest reactive dye, CI Reactive Black 5 , the reaction takes place with the coupling component H-acid .
Another example is the preparation of the reactive dye CI Reactive Orange 107 ( 4 ) by diazotizing parabase ester ( 2 ) and coupling with 3-aminoacetanilide-4-sulfonic acid ( 3 ):
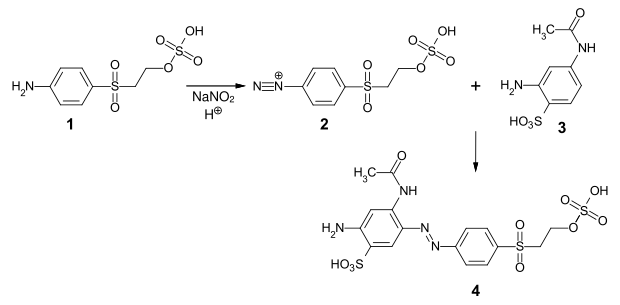
- Synthesis of CI Reactive Orange 107 by diazotization of parabase ester and coupling with 3-aminoacetanilide-4-sulfonic acid
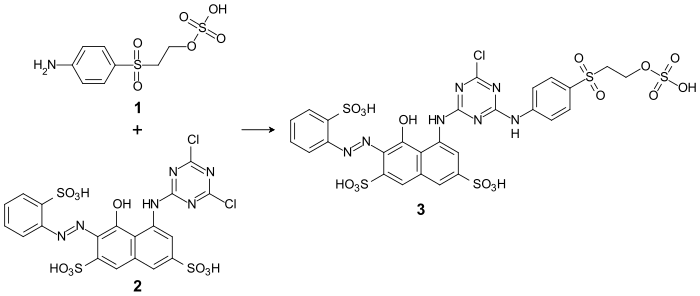
As an aromatic amine, parabase ester reacts with halotriazine derivatives ( cyanuric chloride or cyanuric fluoride , or the corresponding dichloro or difluorine compounds) by substituting the halogen. For example, by reacting parabase ester ( 1 ) with the dichlorotriazinyl dye CI Reactive Red 1 ( 2 ), the bifunctional reactive dye CI Reactive Red 227 ( 3 ):
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2 - [(p-aminophenyl) sulphonyl] ethyl hydrogen sulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on October 20, 2018, is reproduced from a self-classification by distributors .
- ↑ a b Registration dossier for 2 - [(p-aminophenyl) sulphonyl] ethyl hydrogensulphate ( GHS section ) at the European Chemicals Agency (ECHA), accessed on October 22, 2018.
- ^ E. Siegel: Reactive Dyes: Reactive Groups . In: K. Venkataraman (Ed.): The Chemistry of Synthetic Dyes . Volume VI. Academic Press, New York and London 1972, pp. 36 ff .
- ↑ Patent DE19540544 : Ethanol-2- (4-aminophenyl) -sulphonyl hydrogen sulphate compound preparation. Registered on May 7, 1997 , applicant: Dystar Textilfarben GmbH and Co Deutschland KG.
- ↑ patent DE10005550 : Production of azo dyes and pigments. Registered on August 23, 2001 , applicant: Clariant Produkte (Deutschland) GmbH. , Example 3 (Water-soluble reactive dyes / Reactive Orange 107).
- ↑ External identifiers or database links to CI Reactive Red 227, tetrasodium salt : CAS number: 23354-53-2 , EC number: 607-222-2, ECHA InfoCard: 100.133.397 , PubChem : 274107460 , Wikidata : Q73383968 .
- ^ Heinrich Zollinger: Color Chemistry . 3rd, revised edition. Wiley-VCH, Weinheim 2003, ISBN 3-906390-23-3 , pp. 225 ff .