Reactive Black 5
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Reactive Black 5 | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 26 H 21 N 5 Na 4 O 19 S 6 | ||||||||||||||||||
| Brief description |
dark brown powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 991.8 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.21 g cm −3 |
||||||||||||||||||
| Melting point |
> 180 ° C |
||||||||||||||||||
| solubility |
550 g l −1 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
> 5000 mg kg −1 ( LD 50 , rat, female , oral ) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
CI Reactive Black 5 is a bisazo dye of the performance group of reactive dyes , which for dyeing of cotton and wool is used.
Manufacturing
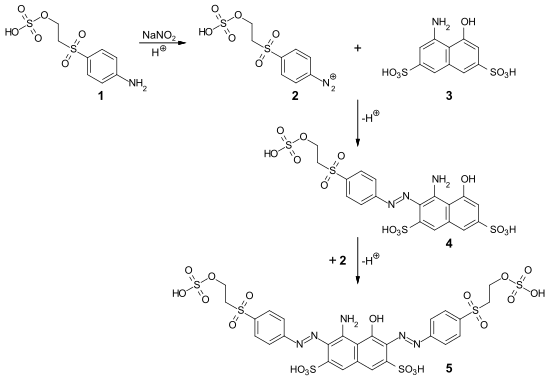
Reactive Black 5 ( 5 ) is synthesized by diazotizing parabase ester ( 1 ) with sodium nitrite to form diazo component 2 and coupling with H acid ( 3 ). First, the two components are converted to the red monoazo dye ( 4 ) at a low pH ( acidic coupling ) . Then, at a higher pH, the diazo component is coupled adjacent to the hydroxyl group of the H-acid ( alkaline coupling ):
Depending on the reaction conditions ( stoichiometry , concentration ), the end product contains proportions of the two isomeric red monoazo dyes in which the azo group is adjacent to the amino group ( 4 ) or to the hydroxy group of the H-acid.
use
Reactive Black 5 is one of the first vinyl sulfone dyes for which a patent was applied for by Farbwerke Hoechst in 1952 and which was marketed under the trade name Remazol Black B from 1957 . It was the first reactive dye with two reactive groups in the molecule. Since the two reactive groups are identical, one speaks of a homobifunctional reactive dye.
With Reactive Black 5, a navy blue to black shade can be achieved on cotton and wool. In combination with red or orange dyes, deep black colors are obtained. Reactive Black 5 is the largest and most widely used reactive dye in the world.
Individual evidence
- ↑ a b c d e f g Data sheet Reactive Black 5 at Sigma-Aldrich , accessed on November 4, 2019 ( PDF ).
- ↑ There is not yet a harmonized classification for this substance . A labeling of CI Reactive Black 5 in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on November 04, 2019, is reproduced from a self-classification by the distributor .
- ↑ Karl Rebsamen: From colorless chemicals to colored textiles . In: Science in chemistry lessons . tape 87 . Friedrich-Verlag, Hanover May 2005, p. 48 .
- ↑ Patent DE965902 : Process for fixing water- soluble organic compounds on substrates with a fibrous structure. Registered on April 3, 1952 , published on September 19, 1957 , applicant: Hoechst AG, inventor: Johannes Heyna, Willy Schumacher.
- ↑ J. Heyna: reactive dyes with vinyl sulfone groups . In: Angewandte Chemie . tape 74 , no. 24 , December 21, 1962, pp. 966 , doi : 10.1002 / anie.19620742403 .
- ↑ a b Entry on Reactive Black 5. In: Römpp Online . Georg Thieme Verlag, accessed on November 5, 2019.