Developing dyes
The developing dyes are water-insoluble azo dyes for dyeing and printing cellulose fibers , which are formed directly on the fiber by coupling a water-soluble coupling component with a water-soluble diazo compound . Since the developing dyes are sometimes colored at low temperatures of 0–10 ° C, they are also known as ice colors. This class of dyes is characterized by very good wet fastness .
Occasionally, mordant dyes and vat dyes , in which the sparingly or insoluble dye is also formed on the fiber, are classified as developing dyes.
history
The first attempts to produce water-insoluble azo dyes directly on cotton fibers go back to 1880. The cotton was impregnated with an alkaline solution of 2-naphthol and then treated with various diazo compounds, for example from aniline , toluidine , xylidine or naphthylamine . The problem, however, was the low affinity of sodium 2-naphtholate. The breakthrough for this class of dyes came in 1912 with the development of 2-hydroxynaphthalene-3-carboxylic acid anilide (Naphthol AS) . In the period that followed, a number of other coupling components were developed with which, in a combination of different diazo components, the so-called real bases , shades from the entire color spectrum from yellow, through orange, scarlet and red, to blue could be achieved. With the development of reactive dyes from 1956, the importance of developing dyes declined.
Chemical properties
Naphthol AS and naphthol AS derivatives, which are soluble in water as an anion and have a good affinity for cellulose, are reacted on the fiber with diazobenzene or diazobenzene derivatives. This produces azo dyes in which the acidity of the hydroxyl group in the o -position to the azo group is greatly weakened. They are therefore almost insoluble in water and have very good wet fastness. The dyeing process takes place in stages. In the first step, the material to be dyed is impregnated with an alkaline solution of the coupling component and optionally dried. The impregnated dyed material is then treated with a diazonium salt solution in the so-called development step. Finally, the dye is rinsed and washed.
Coupling components
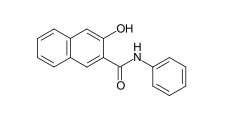
In addition to the most important coupling component, the parent compound Naphthol AS, other Naphthol AS derivatives have been developed, which are different anilides of 2-hydroxynaphthalene-3-carboxylic acid. To achieve yellow nuances, anilides of acetoacetic acid are used (e.g. BCI Azoic Coupling Component 35). With carbazole or dibenzofuran derivatives, for example the anilides of 2-hydroxycarbazole-1-carboxylic acid or 2-hydroxydibenzofuran-3-carboxylic acid, shades of brown can be colored.
According to the system of the Color Index , the coupling components of the developing dyes are referred to as CI Azoic Coupling Component .
Examples:
Diazo components
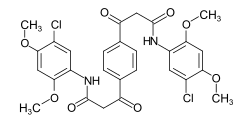
The aromatic amines, which are used as diazo components for developing dyes , are also referred to as real bases , while the corresponding diazonium compounds are real salts .
The diazo compounds of the developing dyes are designated as CI Azoic Diazo Component according to the Color Index .
Examples:
Since the diazotization of aromatic amines in the dye works is sometimes difficult, the following alternative options are available:
- Stabilized diazo
salts : As a rule, diazonium salts cannot be isolated and are explosive in dried form. However, they can be stabilized with suitable counter anions. For example, zinc chloride double salts or naphthalene sulfonates of diazonium compounds are so stable that they can be isolated, dried and stored. These stabilize diazo salts are also known as true salts . - Rapid fast dyes:
The diazonium salts are converted into the trans -diazotates and mixed with the naphthol AS compounds. The alkaline aqueous solution of this mixture is applied to the fiber and developed by adding acid. The addition of acid shifts the diazonium / diazotate equilibrium to the diazonium ion, which then quickly enters into the coupling reaction.
- Rapidogen dyes:
With the rapidogen dyes, the unstable diazonium compounds are converted with a primary or secondary aliphatic or aromatic amine into a diazoamino compound (triazene) , which is quite stable at a higher pH value. These are then applied to the cellulose in a mixture with a naphthol AS. On treatment with acid, the diazonium compound is released again and couples to the dye.
- Rapidazole dyes:
With these dyes, the diazonium salt is stabilized as aryldiazosulfonate. These are applied to the fiber in a mixture with naphthol AS derivatives and can be steamed, preferably in the presence of an oxidizing agent, such as. B. sodium chromate , are developed.
Individual evidence
- ↑ Entry on developing dyes . In: Römpp Online . Georg Thieme Verlag, accessed on January 25, 2019.
- ↑ a b M. Satake, Y. Mido: Chemistry of Color . Discovery Publishing House, New Delhi 1995, ISBN 81-7141-276-9 , pp. 68 ff . ( limited preview in Google Book search).
- ↑ a b c d Paul Rys, Heinrich Zollinger: Guide to dye chemistry . Ed .: Wilhelm Foerst, Helmut Grünewald (= chemical pocket books . Volume 13 ). Verlag Chemie, Weinheim 1970, p. 63 ff .
- ↑ Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 375 ( limited preview in Google Book search).
- ^ RLM Allen: Color Chemistry . Springer, New York 1971, ISBN 978-1-4615-6665-6 , pp. 101 ( limited preview in Google Book search).