Naphthalene-1,5-disulfonic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
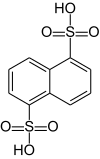
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Naphthalene-1,5-disulfonic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 1 0 H 8 O 6 S 2 | |||||||||||||||
| Brief description |
white odorless solid (tetrahydrate) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 288.3 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| Vapor pressure |
51 hPa (50 ° C) |
|||||||||||||||
| solubility |
soluble in water (1030 g l −1 at 20 ° C, tetrahydrate)
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Naphthalene-1,5-disulfonic acid is a chemical compound from the group of sulfonic acids . The compound is also known as Armstrong's acid after the British chemist Henry Edward Armstrong .
Extraction and presentation
Naphthalene-1,5-disulfonic acid can be obtained by reacting naphthalene or naphthalene-1-monosulfonic acid with fuming sulfuric acid at below 25 ° C. The reaction also produces naphthalene-1,6-disulfonic acid, naphthalene-1,3,6-trisulfonic acid and naphthalene-1,3,7-trisulfonic acid. It can also be obtained by reacting naphthalene with sulfur trioxide in sulfuric acid at low temperatures.
properties
As a tetrahydrate, naphthalene-1,5-disulfonic acid is a white, odorless solid that is soluble in water.
use
Naphthalene-1,5-disulfonic acid is used as an intermediate in the manufacture of dyes. In this process, diazonium salts are stabilized with naphthalene-1,5-disulfonate as the counteranion, so that they can be isolated, dried and stored. The stabilized diazonium salts are used as so-called true salts in developing dyes . Example: Real Red Salt TR (CI Azoic Diazo Component 11)
Individual evidence
- ↑ a b c d e f g h Data sheet naphthalene-1,5-disulfonic acid (PDF) from Merck , accessed on November 5, 2018.
- ↑ a b c d e David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 418 ( limited preview in Google Book search).
- ↑ a b Data sheet 1,5-Naphthalenedisulfonic acid tetrahydrate, 97% from Sigma-Aldrich , accessed on November 5, 2018 ( PDF ).
- ↑ Alexander Senning: Elsevier's Dictionary of Chemoetymology The Whys and Whences of Chemical Nomenclature and Terminology . Elsevier, 2006, ISBN 978-0-08-048881-3 , pp. 30 ( limited preview in Google Book search).
- ^ Houben-Weyl Methods of Organic Chemistry Vol. IX, 4th Edition Sulfur, Selenium, Tellurium Compounds . Georg Thieme Verlag, 2014, ISBN 3-13-180544-7 , p. 477 ( limited preview in Google Book search).
- ↑ Hans Theodor Bucherer: Textbook of color chemistry - including the extraction and processing of the tar as well as the methods for the representation of the preliminary and intermediate products . Springer-Verlag, 2013, ISBN 978-3-662-33911-4 , pp. 107 ( limited preview in Google Book search).
- ↑ Google Patents: EP0000155A1 - Continuous production of naphthalene-1-sulfonic acid and 1,5-disulfonic acid - Google Patents , accessed November 5, 2018.
- ^ SVS Rana: Biotechniques Theory & Practice . Rastogi Publications, 2008, ISBN 978-81-7133-886-3 , pp. 57 ( limited preview in Google Book search).
- ↑ Data sheet Fast Red TR Salt 1,5-naphthalenedisulfonate salt from Sigma-Aldrich , accessed on January 29, 2019 ( PDF ).
