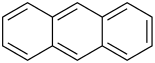
Anthracene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Anthracene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 10 | |||||||||||||||
| Brief description |
white to yellowish leaves with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.25 g cm −3 |
|||||||||||||||
| Melting point |
217 ° C |
|||||||||||||||
| boiling point |
340 ° C |
|||||||||||||||
| Vapor pressure |
8 mPa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : persistent, bioaccumulative and toxic ( PBT ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Anthracene [ antraˈt͡seːn ] (from the Greek anthrax , 'coal'), also paranaphthalene or anthracene is a colorless crystalline solid that easily sublimates . It is a polycyclic aromatic hydrocarbon and organic semiconductor with the empirical formula C 14 H 10 , which is made up of three fused benzene rings.
history
Anthracene was first isolated from tar in 1832 by Auguste Laurent and Jean-Baptiste Dumas . Laurent also made anthraquinone and phthalic acid for the first time in 1836 by oxidizing the anthracene .
Occurrence
On earth, anthracene is found in coal tar . Oxidized anthracene derivatives - mostly anthraquinones - are common in organisms. Positively charged anthracene molecules have been detected in meteorites as well as recently by the Royal Astronomical Society in interstellar matter . The most complex molecule discovered in interstellar space before anthracene was naphthalene .
Extraction and presentation
Anthracene is obtained industrially from coal tar . The synthetic production takes place by pyrolysis of 2-methylbenzophenone or by Friedel-Crafts-alkylation of 2-bromobenzyl bromide . Alternatively, it can also be prepared by reducing anthraquinone , which in turn is accessible by the Diels-Alder reaction of p -benzoquinone with 1,3-butadiene or by Friedel-Crafts acylation of benzene with phthalic anhydride in the presence of aluminum chloride . The latter was the original synthesis invented by Richard Anschütz in 1883 . The most commonly used reducing agents for anthraquinone are:
- Zinc in an alkaline environment,
- amalgamated aluminum in secondary alcohols ,
- Tin (II) chloride and HCl in acetic acid
- Sodium borohydride
properties
Anthracene crystallizes in colorless to yellowish flakes, which fluoresce violet and sublime easily . They melt at 216.3 ° C and boil at 340 ° C. Anthracene is almost insoluble in water (about 0.1 mg / l at 25 ° C), sparingly soluble in ethanol (15 g / l) and diethyl ether , and well soluble in boiling benzene . Anthracene only smells very weakly aromatic and is almost odorless. Since only one six-membered ring in anthracene contains an -electron sextet , it is quite reactive, more reactive than the isomer phenanthrene , which, in contrast to anthracene, has two rings with an -electron sextet. In particular, positions 9 and 10 are ideal points of attack z. B. for oxidations, by which anthracene can be converted into anthraquinone , for example. Anthracene dimerizes when exposed to UV light ; however, the dimer breaks down again as soon as the heat is applied.

Since there are two different mesomeric boundary structures for anthracene according to the incremental system, these must be taken into account when calculating the mesomeric energy and the mean value of the enthalpy of formation is to be calculated before the difference to the actual enthalpy of formation is drawn.
The flash point is 121 ° C, the ignition temperature 540 ° C. The lower explosion limit (LEL) in air is 0.6% by volume (45 g / m 3 ). Anthracene is hazardous to water (WGK 2). The standard enthalpy of formation in the solid phase is 121 ± 10 kJ mol −1 , in the gas phase 223 ± 10 kJ mol −1 . The standard enthalpy of combustion in the solid phase is −7061 ± 10 kJ mol −1 .
use
Anthracene is almost exclusively processed into anthraquinone , which is the starting point for the anthraquinone dyes and thus the basis for the alizarin and indanthrene dyes . Anthracene is also used as a base for the production of pesticides and tannins . The anthracene derivative dithranol is the oldest active ingredient that was developed for the treatment of psoriasis .
nomenclature
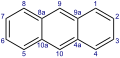
According to the IUPAC nomenclature, anthracene has a special position numbering of the carbon atoms, which is derived from the (special) numbering of its isomer phenanthrene . This numbering is partially transferred to heterocyclic compounds with the same basic structure (in which only carbon atoms are replaced by other atoms). An example is 4-azaphenothiazine (pyridobenzothiazine, pyrido [3,2- b ] benzothiazine) and derivatives thereof such as prothipendyl . There, the usual dominant role of the hetero-atoms in the numbering does not apply .
Phenanthrene with special numbering
The standard numbering of anthracene would match up to position 4 with the one shown here, but then continue all around without "jumps". With phenanthrene, on the other hand, the standard numbering would have to run in the opposite direction and start at position 4 shown here.
Individual evidence
- ↑ a b c d e f Entry on anthracene in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e Entry on anthracene. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Wolfgang Gerhartz (Ed.): Ullmann's Encyclopedia of Industrial Chemistry. Vol. A. / 2nd, 5th ed., VCH, Weinheim 1985, ISBN 3-527-20102-5 .
- ↑ Data sheet anthracene for synthesis (PDF) from Merck , accessed on May 31, 2013.
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ Scinexx: Complex hydrocarbon discovered in space . News from June 23, 2010.
- ↑ Gary W. Breton, Xoua Vang: Photodimerization of anthracenes: A [4ps + 4ps] Photochemical cycloaddition. In: Journal of Chemical Education . 75 (1), 1998, p. 81, doi: 10.1021 / ed075p81 .
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ a b Entry on Anthracene (Condensed phase thermochemistry data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2019.
- ↑ Entry on Anthracene (Gas phase thermochemical data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2019.
- ↑ Bertram Philipp, Peter Stevens: Fundamentals of industrial chemistry. VCH Verlagsgesellschaft mbH, 1987, ISBN 3-527-25991-0 , p. 183.
- ↑ Ulrich Mrowietz and Gerhard Schmid-Ott: Psoriasis - What you always wanted to know about psoriasis. 3rd updated edition, Karger Verlag, 2012, ISBN 978-3-8055-9396-0 , p. 24.
- ↑ IUPAC Nomenclature of Organic Chemistry: Rule A-22.5 (see examples).