4-azaphenothiazine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
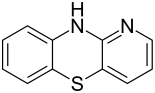
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-azaphenothiazine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 8 N 2 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 200.26 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
114-115 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
In chemistry, 4-azaphenothiazine belongs to the group of heterocycles and is an intermediate product in the synthesis of a large number of medicinal products .
history
The first synthesis succeeded in 1958 by a Smiles rearrangement with subsequent cyclization .
Extraction and presentation
Melting N - (pyrid-2-yl) aniline with sulfur in the presence of catalytic amounts of iodine gives 4-azaphenothiazine. Alternatively, 4-azaphenothiazine can also be prepared starting from 2-chloropyridine and 2-aminothiophenol .
properties
4-Azaphenothiazine can be alkylated on the secondary amino group (NH) by reaction with haloalkanes in a basic medium. The reaction of 4-azaphenothiazine with phosgene gives 4-azaphenothiazine-10-carboxylic acid chloride, which reacts with alcohols to form urethanes , with thiols to form thiocarbamic acid esters and amines to form urea derivatives .
use
4-Azaphenothiazine is used as a starting material for the drugs prothipendyl (Dominal ® ), isothipendyl (Andantol ® ) and pipacetate (Selvigon ® / Theratuss).
Individual evidence
- ↑ a b W. Schuler , H. Klebe: 4-Azaphenothiazines and their 10-aminoalkyl derivatives , in Liebigs Ann. 1962 , 653 , 172-180; doi: 10.1002 / jlac.19626530120 .
- ↑ a b data sheet 10H-PYRIDO (3,2-B) (1,4) BENZOTHIAZINE from Sigma-Aldrich , accessed on May 12, 2011 ( PDF ).
- ↑ HL Yale, F. Sowinski: 10- (Dialkylaminoalkyl) -pyrido [3,2-b] [1,4] benzothiazine (1-Azaphenothiazine) and Related Compounds , in: J. Am. Chem. Soc. 1958 , 80 , 1651-1654; doi: 10.1021 / ja01540a035 .
- ^ B. Kutscher, HR Dieter, H.-G. Trömer, B. Bartz, J. Engel, A. Kleemann : New synthesis of 4-azaphenothiazine , in: Liebigs Ann. 1995 : 591-592; doi: 10.1002 / jlac.199519950381 .
- ↑ W. Schuler , H. Klebe, A. von Schlichtegroll: Derivatives of 4-azaphenothiazine-10-carboxylic acid , in: Liebigs Ann. 1964 , 673 , 102-112; doi: 10.1002 / jlac.19646730114 .
- ^ A. von Schlichtegroll, in: Arzneimittel-Forschung 1957 , 7 , 237.
- ^ Wilhelm A. Schuler, Hans Klebe, Ansgar V. Schlichtegroll: Syntheses of 4-aza-phenothiazines, II. Derivatives of 4-aza-phenothiazine-10-carboxylic acid . In: Justus Liebig's Annals of Chemistry . tape 673 , no. 1 , May 4, 1964, pp. 102-112 , doi : 10.1002 / jlac.19646730114 ( PDF ).
