Sudan dyes
Sudan dyes are synthetically produced solvent dyes , whereby the yellow, orange and red types are azo dyes , the blue types are anthraquinone dyes and the green types are mixtures of azo and anthraquinone dyes. They are soluble in hydrocarbons , oils , fats and waxes and are therefore used for dyeing them.
etymology
In connection with cases of abuse, the Sudanese embassy made an inquiry to the Food Standards Agency (FSA) because the impression could be given that the dyes are manufactured in Sudan and could damage the country's reputation. In the course of the discussion, the question of the origin of the word could not be answered by either the FSA or the official side of Sudan. The dyes have been known since 1883, with Sudan-III animal experiments were carried out at least from 1886. Answering the question would be a matter of the history books, according to the FSA. One theory of naming states that both names derive from the Arabic word "Sudd" ("blockade" / "barrier"), which denotes the papyrus swamp with the same name in South Sudan . Some other dyes are also named after African regions or states, e.g. B. Congo red , without the reference being completely clarified.
Substances
| Name Color Index designation |
colour | Structural formula |
|---|---|---|
|
Sudan I Solvent Yellow 14 |

|
|
|
Sudan II Solvent Orange 7 |

|
|
|
Sudan III Solvent Red 23 |
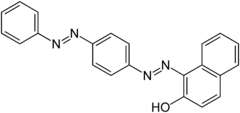
|
|
|
Sudan IV Solvent Red 24 |

|
|
|
Sudan Blue II Solvent Blue 35 |

|
|
|
Sudangelb G Solvent Yellow 16 |

|
|
|
Sudan Orange G Solvent Orange 1 |

|
|
|
Sudan Red B Solvent Red 25 |

|
|
|
Sudan red G Solvent Red 1 |
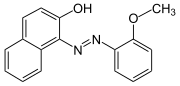
|
|
|
Sudan Red 7B Solvent Red 19 |
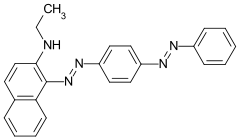
|
|
|
Sudan Black B Solvent Black 3 |

|
Various Sudans with azo group (R 1 –N = N – R 2 ) are known: For example, the yellow Sudan I , the orange Sudan II , the red Sudan III and Sudan IV with a scarlet color. Sudan I and II as well as Sudan III and IV are structurally very similar and only differ in terms of two additional methyl groups.
properties
Sudan dyes that contain the functional group R-N = N-R 'belong to the azo compounds. R and R 'can either be an alkyl or an aryl group , the aryl groups being more stable because of their aromaticity. Both the phenyl and the naphthanol groups are aromatic ring systems. The sp2 hybridized nitrogen atoms in the azo group have a p orbital that share a pair of π electrons that connect the aromatic ring systems to form a fully conjugated system. This conjugation enables the molecule to absorb light in the visible range, making it useful as a dye, with longer conjugated systems absorbing longer wavelengths. Such compounds are known to exist as a pair of tautomers :
use
Sudan dyes are used in histology for staining, with the fat specificity of the staining differing between the various dyes. So it is not Sudan Black B, but Sudan Red 7B that stains most of the lipids. But it also has the greatest fat unspecificity. Sudan IV shows the lowest lipid unspecificity, followed by scarlet R med and Sudan black B. Individual solvents influence the color width of Sudan black B. Fats are mainly derived from Sudan black B-diacetin, from the chromatographically blue fraction of Sudan black B ( ethanol ) and from Sudan black B. - Propylene glycol recorded. Protein substances are particularly tinged with Sudan black B-propylene glycol; Colloidal Sudan black B-ethanol, Sudan black B-triethyl phosphate and the chromatographically blue fraction of Sudan black B (ethanol) then follow at a distance. Polysaccharides are stained most strongly by the blue chromatographic fraction of Sudan black B (ethanol), Sudan black B-propylene glycol and the colloidal Sudan black B-ethanol. Despite these objections, the Sudan dyes can be used in routine histology to represent “free” fats if ethanol or isopropanol is used as the solvent.
In the European Union , they have not been approved as a food additive since 1995 because they can be broken down in the body into amines , some of which are carcinogenic . Since the EU-wide controls introduced in mid-2003, Sudan dyes have been repeatedly detected in imported products. While the controls previously concentrated on chili powder , turmeric and native palm oil are now also being targeted. The substances can also be found in products containing tomatoes and paprika, such as pesto .
Sudan I and IV are also used for Semtex dyeing.
The German Mineral Oil Tax Act , in its original version from 1964, prescribed the dye Sudan Red 7B (Solvent Red 19) for marking tax-privileged mineral oils ( e.g. heating oil ) . The compound is a powder dye and its processing has known disadvantages, e.g. B. Risk of exposure in production and processing, high consumption of solvents for the manufacture of the "packages" and time-consuming dissolving processes. With the approval of modified dyes, the Mineral Oil Tax Act 1977 was adapted to the current technical and industrial hygiene requirements. Liquid dyes have been used and processed since then. According to the current ordinance and specification of the Federal Ministry of Finance in the Mineral Oil Tax Act and TRGS 614 (restrictions on use for azo dyes that can be split into carcinogenic aromatic amines) for marking mineral oil, a liquid dye is used that consists of two components (CAS numbers 56358-09-9 N - (2-Ethylhexyl) -1 - ({2-methyl-4 - [(2-methyl-phenyl) azo] phenyl} azo) naphthayl-1-amine and 57712-94-4 1 - ({2-methyl -4 - [(2-methylphenyl) azo] phenyl} -azo) - N -tridecylnaphthayl-2-amine is made). The dye Solvent Red 215 (CAS No. 85203-90-3) is also used for mineral oil exports. The liquid dye mentioned (CAS number 56358-09-9) is also imported into the Federal Republic of Germany for the same area of application. In addition, to the knowledge of the AGS, mineral oils are imported from neighboring EC countries that are colored with the azo dyes Sudan IV , Solvent Red 164 (92257-31-3) or Solvent Red 215 (85203-90-3) in accordance with the local regulations . Since 2002 the EU has uniformly switched to labeling with the yellow marker Solvent Yellow 124 , the analytical detection of which is much less laborious to carry out. Since Solvent Yellow 124 only insignificantly colors the heating oil, 4.1 to 4.9 mg / l of the azo dye mixture Sudan Red M 462 are added. Sudan red M 462 consists of the above-mentioned red dyes N -ethyl-hexyl- (tolylazotolylazo) naphthyl-2-amine and N- tridecyl-1- (tolylazotolylazo) naphthyl-2-amine and can split off the carcinogenic o -toluidine .
abuse
In December 2006, the head of Guangzhou Tianyang Foodstuffs Company in southern China , a company that sells food in China and abroad, was sentenced to 15 years 'imprisonment and his deputy was sentenced to 10 years' imprisonment for using the dye in Sudan Red (Sudan III) Had mixed in chili oil and powder . Between April 2002 and March 2005 they are said to have made well over half a million US dollars in profit with this method . British food inspectors also found the dye Sudangelb (Sudan I) in Worcestershire sauce made in England on February 18, 2005 .
Others
See also
Individual evidence
- ↑ https://www.jbc.org/content/13/1/71.full.pdf published in Archives Italiennes de Biologie issue 26. 1896
- ↑ https://www.archivesofpathology.org/doi/full/10.1043/0003-9985(2001)125%3C0250%3ACR%3E2.0.CO%3B2
- ↑ http://www.chinadaily.com.cn/english/doc/2005-03/12/content_424157.htm
- ↑ http://news.bbc.co.uk/2/hi/africa/4318419.stm
- ↑ Clayden, JG, N. Warren, S. Wothers, P., Organic Chemistry 1st ed .; Oxford University Press: 2001.
- ↑ Herbert J. Schott, Wilhelm Schoner: Contribution to the fat specificity of Sudan black B and other red Sudan dyes in pure substances. In: Histochemistry . 5, 1965, p. 154, doi : 10.1007 / BF00285509 .
- ↑ Federal Institute for Risk Assessment : Sudan I to IV dyes in food (PDF; 158 kB), BfR opinion of November 19, 2003.
- ^ Alexander Beveridge: Forensic Investigation of Explosions , CRC Press, 1998, ISBN 0-7484-0565-8 , p. 297 ( limited preview in Google book search).
- ↑ berufssicherheit.de: Annex 2 TRGS 614, Dyes for marking mineral oils - Library - berufssicherheit.de ( Memento of the original from November 12, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed November 11, 2016.
- ^ The entire consumption tax law 2011 directives, laws, regulations; Text collection . Walhalla Fachverlag, 2011, ISBN 978-3-8029-1912-1 , p. 161 ( limited preview in Google Book search).
- ↑ : German Statutory Accident Insurance BK-Report Aromatic Amines - A working aid in occupational disease determination procedures - Report of the accident insurance institutions and the IFA - ( Memento of the original from November 12, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed November 11, 2016.
- ↑ Chinese convicted of adding color to food ( memento from July 18, 2012 in the web archive archive.today ) Page 1 - AFP from December 7, 2006.
- ^ Federal Office of Public Health (FOPH): Sudan ( memento of August 17, 2009 in the Internet Archive ), accessed on July 29, 2008.
- ↑ Werner Baltes, Reinhard Matissek: Food chemistry . Springer-Verlag, 2011, ISBN 978-3-642-16539-9 , pp. 325 ( limited preview in Google Book Search).


