Mauveine

The mauveins are a group of basic azine dyes in the eponymous color mauve . Chemically, it is structurally very similar structure phenazine - derivatives that the Safraninen be counted. The mixture of the individual compounds formed during the preparation of the dye serves (e) primarily as a textile dye. As such, it is also known under the names aniline purple and perkin purple .
history
Mauvein was invented in 1856 by William Henry Perkin, who was only 18 at the time, while trying to synthesize quinine . He made the dye from aniline , which was oxidized with potassium dichromate . The aniline he used as a starting material, however, contained considerable amounts of o- and p - toluidine , so that the product obtained was a mixture of mauvein and pseudomauvein. According to legend, Perkins' first dyed piece of fabric was his sister's previously white blouse, which then shone in a bright mauve color.
Representative
The mauveins differ in the number and position of the methyl groups. Up to 13 different individual compounds were detected in historical dye samples, the main components being mauvein A, B, B2 and C, i.e. structures with 26 to 28 carbon atoms.
| Mauveine | ||||
| Surname | Mauvein A | Mauvein B | Mauvein B2 | Mauvein C |
| Structural formula (cation) |
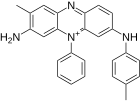
|

|

|

|
| other names | 3-Amino-2,9-dimethyl-5-phenyl-7- ( p -tolylamino) phenazinium acetate |
|||
| CAS number | 153343-19-2 (cation) 153343-20-5 ( acetate ) |
946132-01-0 (cation) | ||
| 6373-22-4 (mixture) | ||||
| PubChem | 407617 | |||
| Molecular formula (cation) | C 26 H 23 N 4 + | C 27 H 25 N 4 + | C 27 H 25 N 4 + | C 28 H 27 N 4 + |
| Molar mass (cation) | 391.19 g mol −1 | 405.21 g mol −1 | 405.21 g mol −1 | 419.22 g mol −1 |
properties
Mauvein ( CAS : 6373-22-4) is an almost black powder that is insoluble in water but soluble in ethanol . It is resistant to alkalis and acids . A hydrochloride forms greenish, shiny prisms.
It has a high affinity for fibers, especially cotton and silk , which are brilliantly dyed.
use

Until the late 19th and early 20th century, the English were Penny - Stamps tinged with mauve. Today mauvein is no longer used as a coloring agent.
Individual evidence
- ↑ The Great Chronicle of World History: The Great Chronicle of World History 13. Industrialization and national awakening: 1849-1871: Volume 13 , p. 114, 1st edition, Chronik Verlag, 2008, ISBN 3-577-09073-1 .
- ^ Karl Huebner 150 Years of Mauvein , Chemistry in Our Time, Volume 40, 2006, pp. 274–275.
- ↑ a b Micaela M. Sousa, Maria J. Melo, A. Jorge Parola, Peter JT Morris, Henry S. Rzepa, J. Sérgio Seixas de Melo: A Study in Mauve: Unveiling Perkin's Dye in Historic Samples. In: Chemistry - A European Journal. 14, 2008, pp. 8507-8513, doi : 10.1002 / chem.200800718 .
- ↑ Otto Meth-Cohn, Mandy Smith: What did WH Perkin actually make when he oxidized aniline to obtain mauveine ?. In: Journal of the Chemical Society, Perkin Transactions 1. 1994, p. 5, doi : 10.1039 / P19940000005 .
- ↑ a b c A. Kekulé : Chemistry of the benzene derivatives or the aromatic substances, Volume 1 , Verlag Ferdinand Enke, Erlangen, 1867.