Indanthrone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
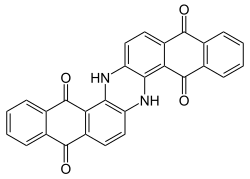
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Indanthrone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 28 H 14 N 2 O 4 | ||||||||||||||||||
| Brief description |
blue solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 442.42 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
practically insoluble in water (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Indanthrone is a chemical compound that is commercially produced as an anthraquinone dye in the colorant industry and is used in research and development because of its catalytic and optical properties. The structure of the molecule is derived from the polycyclic aromatic hydrocarbon naphtho [2,3- c ] pentaphene. Four crystal modifications (α, β, γ, δ) are known, of which the α modification is the most stable form.
The dye is known as the first representative of the brand name Indanthrene , introduced by BASF in 1901 - an acronym for indigo made from anthracene .
presentation
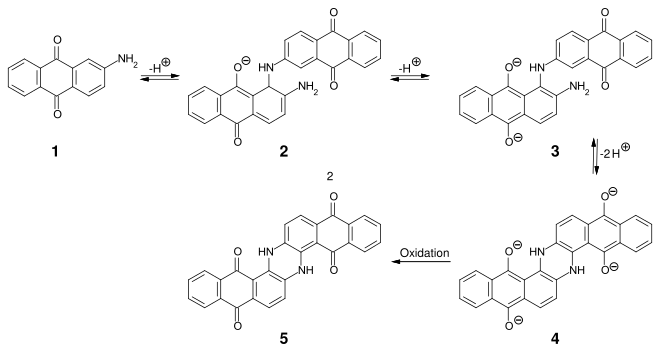
Indanthrone was the first synthetic anthraquinone- based vat dye . The synthesis was developed by the BASF chemist René Bohn :
By dimerizing 2-aminoanthraquinone ( 1 ) under strongly alkaline conditions at 220–235 ° C, intermediate 3 is obtained in two steps , which is intramolecularly cyclized and oxidized to indanthrone 5 .
properties
- Organic semiconductor from the class of conjugated molecules .
- non-linear light absorber
- Colorant (blue pigment )
- Temperature resistance: 200 ° C
use
Organic semiconductor
As an organic semiconductor , indanthrone z. B. as a photocatalyst for oxygen production from water using solar energy . In addition, indanthrone carry out its capacity as a non-linear light absorber as a so-called optical limiter (Engl. Optical limiter ) z. B. use in laser protection filters.
Colorants
Indanthrone is mainly used as a blue pigment (CI Pigment Blue 60) in vat dyeing under the name CI Vat Blue 4. Indanthron is a synthetically produced vat dye with the highest fastness properties for dyeings and prints, especially for cellulose- based textile fibers . The fibers dyed with indanthrene meet the highest demands and have excellent washing , cooking , light , weather and chlorine fastness properties .
trademark
Indanthren has been a trademark of the textile industry for lightfast and washfast dyed textiles since 1922 , regardless of the type of dye used.
Individual evidence
- ↑ Entry on CI 69800 in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b W. Herbst, K. Hunger: Industrial Organic Pigments. Production, properties, application . 2nd edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-28744-2 .
- ↑ a b c Entry on 6,15-dihydroanthrazine-5,9,14,18-tetron in the GESTIS substance database of the IFA , accessed on February 28, 2017(JavaScript required) .
- ↑ D. Thetford et al .: Intermolecular interactions of flavanthrone and indanthrone pigments . In: Dyes and Pigments 67, 2005, pp. 139-144, doi : 10.1016 / j.dyepig.2004.11.002 .
- ↑ Entry on indanthrone pigments. In: Römpp Online . Georg Thieme Verlag, accessed on January 16, 2013.
- ↑ Indanthren is a registered trademark of the DyStar Colors Distribution, see entries at DPMAregister .
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 255 ( limited preview in Google Book search).
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 289 ( limited preview in Google Book search).
- ^ CA Linkous, DK Slattery: Solar photocatalytic hydrogen production from water using a dual bed photosystem . In: Proceedings of the 2000 Hydrogen Program Annual Review, Volume I . ( PDF ).
- ↑ DK Slattery et al .: Semiempirical MO and Voltammetric estimation of ionization potentials of organic pigments. Comparison to gas phase ultraviolet photoelectron spectroscopy . In: Dyes and Pigments 49, 2001, pp. 21-27.
- ↑ Y.-P. Sun, JE Riggs: Organic and inorganic optical limiting materials. From fullerenes to nanoparticles . In: International Reviews in Physical Chemistry . 18, No. 1, 1999, pp. 43-90.
- ↑ See for example Sandra Schaumann-Eckel, Heribert Rampf: More Success in Chemistry: Organic Chemistry , mentor, Munich 2010, p. 91.