Benzene dicarbonitrile
| Benzene dicarbonitrile | ||||||
| Surname | Benzene-1,2-dicarbonitrile | Benzene-1,3-dicarbonitrile | Benzene-1,4-dicarbonitrile | |||
| other names |
o -Benzenedinitrile, phthalic acid dinitrile, phthalonitrile , 1,2-dicyanobenzene |
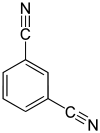
m -Benzenedinitrile, isophthalic acid dinitrile, isophthalonitrile, 1,3-dicyanobenzene |
p -Benzenedinitrile, terephthalic acid dinitrile, terephthalodinitrile, 1,4-dicyanobenzene |
|||
| Structural formula |

|
|

|
|||
| CAS number | 91-15-6 | 626-17-5 | 623-26-7 | |||
| PubChem | 7042 | 12276 | 12172 | |||
| Molecular formula | C 8 H 4 N 2 | |||||
| Molar mass | 128.13 g mol −1 | |||||
| Physical state | firmly | |||||
| Brief description | beige crystalline odorless powder |
colorless crystalline powder with a smell of bitter almonds |
colorless crystals with a smell of bitter almonds |
|||
| Melting point | 138-141 ° C | 162-163 ° C | 224-227 ° C | |||
| boiling point | 288 ° C | |||||
|
Solubility in water |
0.56 g l −1 (25 ° C) | 0.7 g l −1 (20 ° C) | 0.08 g l −1 (23 ° C) | |||
|
GHS labeling |
|
|
|
|||
| H and P phrases | 301 + 311 + 331-412 | 302 + 332 | 315-319-335 | |||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||
| 273-501 | no P-phrases | 261-305 + 351 + 338 | ||||
| MAK | Switzerland: 5 mg m −3 ( inhalable dust ) |
|||||
| LD 50 | 30 mg kg −1 (rat, oral) |
860 mg kg −1 (rat, oral) |
> 6400 mg kg −1 (rat, oral) |
|||
The Phthalonitrile form a in the chemical substance group consisting of a benzene ring with two appended nitrile groups consist (-CN). Their different arrangements ( ortho , meta or para ) result in three constitutional isomers with the empirical formula C 8 H 4 N 2 .
The common names (phthalic acid dinitrile, isophthalic acid dinitrile, terephthalic acid dinitrile) are derived in a corresponding manner from the benzene dicarboxylic acids ( phthalic acid , isophthalic acid , terephthalic acid ).
history
The first presentation of phthalonitrile was reported by Johannes Pinnow in 1896 . It was discovered as a by-product in the reaction between orthoamidobenzonitrile hydrochloride , sodium nitrite and hydrochloric acid to synthesize orthodicyanodiazoamidobenzene . The first direct method of making phthalonitrile was introduced in 1907 with the reaction of phthalamide with acetic anhydride . Although the initial yields were small, it became the forerunner of today's large-scale synthesis processes.
presentation
Phthalonitrile may be in a single-step process by ammoxidation of o -xylene of a in the presence of vanadium - antimony oxide - catalyst at high temperatures (480 ° C) are obtained.
Terephthalonitrile can also be obtained by ammoxidation of p- xylene .
Three other methods are described for the production of phthalonitrile:
- Of phthalic anhydride and ammonia is phthalimide prepared which when further addition of ammonia to phthalamide is reacted. This is treated in pyridine or chlorobenzene as a solvent with dehydrating agents such as phosphorus pentachloride , whereby phthalonitrile is formed.
- Using bauxite as a catalyst, phthalic anhydride and ammonia are allowed to react in the gas phase, phthalonitrile being formed in one step.
- By reacting ammonia with a xylene mixture, phthalonitrile, isophthalonitrile and terephthalodinitrile are obtained. From 1935 to 1966, phthalonitrile was manufactured from phthalic anhydride at BASF AG; from 1966, it was manufactured using o- xylene by ammoxidation at temperatures of up to 500 ° C.
properties
Terephthalonitrile has the highest melting point due to the greatest molecular symmetry. o -Phthalonitrile is readily soluble in alcohol, ether and chloroform and very soluble in benzonitrile .
use
Phthalonitrile is used in the manufacture of phthalocyanine dyes and is found in electrophotographic materials.
Phthalonitrile is also used in the manufacture of pesticides, as a stabilizing additive for aircraft fuels, and in the rubber industry. It is also used as a starting product for the production of optical brighteners and sensitizers for photography.
Web links
Individual evidence
- ↑ a b c d Entry for CAS no. 91-15-6 in the GESTIS substance database of the IFA , accessed on July 28, 2017(JavaScript required) .
- ↑ a b c d e Entry for CAS no. 626-17-5 in the GESTIS substance database of the IFA , accessed on July 28, 2017(JavaScript required) .
- ↑ a b c d Entry for CAS no. 623-26-7 in the GESTIS substance database of the IFA , accessed on July 28, 2017(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 626-17-5 or 1,3-phthalic acid dinitrile ), accessed on February 12, 2020.
- ↑ Johannes Pinnow, C. Sämann: About derivatives of o-amidobenzonitrile ; in: Reports of the German Chemical Society ; 1896 ; 29 (1); Pp. 623-632; doi : 10.1002 / cber.189602901118 .
- ↑ A. Braun, J. Tcherniac: About the products of the action of acetic anhydride on phthalamide ; in: Reports of the German Chemical Society ; 1907 ; 40 (2); Pp. 2709-2714; doi : 10.1002 / cber.190704002202 .
- ↑ Peter M. Lorz, Friedrich K. Towae, Walter Enke, Rudolf Jäckh, Naresh Bhargava, Wolfgang Hillesheim: Phthalic Acid and Derivatives . Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim 2002; doi : 10.1002 / 14356007.a20_181.pub2 .
- ↑ a b c Toxicological evaluation of o-phthalonitrile (PDF) at the professional association for raw materials and chemical industry (BG RCI), accessed on August 22, 2012.



