Phthalamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
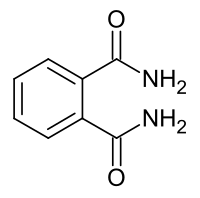
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phthalamide | |||||||||||||||
| Molecular formula | C 8 H 8 N 2 O 2 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 164.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
223 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in water and ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phthalamide is a chemical compound from the group of amides .
Extraction and presentation
Phthalamide can be produced by the reaction of ammonia with phthalic acid diester or phthalimide .
properties
Phthalamide is a white solid that is sparingly soluble in water.
use
Phthalamide is used to make other chemical compounds (e.g. phthalonitrile ).
Individual evidence
- ↑ a b c d e f data sheet Phthalamide, 97% from AlfaAesar, accessed on February 24, 2020 ( PDF )(JavaScript required) .
- ↑ a b David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 38 ( limited preview in Google Book search).
- ↑ a b Lexicon of Chemistry: Phthalamid - Lexicon of Chemistry , accessed on February 24, 2020.