Phthalocyanine green
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
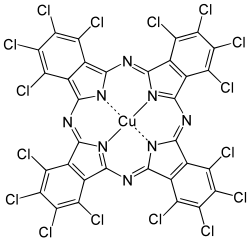
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phthalocyanine green | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CuC 32 Cl 16-n H n N 8 (n = 0; 1; 2) | |||||||||||||||
| Brief description |
green, odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 1127.18 g mol −1 , 1092.74 g mol −1 , 1058.30 g mol −1 (depending on n) | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.00 g cm −3 |
|||||||||||||||
| Melting point |
480 ° C (from 350 ° C decomposition) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phthalocyanine Green is a polychloropper phthalocyanine . It is a synthetic dark green pigment and, according to its chemical structure, belongs to the phthalocyanines . The complex organic compound is derived from phthalic acid . It is listed under CI Pigment Green 7 in the Color Index .
history
Phthalocyanine Green (Pigment Green 7) was first produced on a commercial scale in 1938, three years after phthalocyanine blue was introduced commercially. The brominated variant, Pigment Green 36, was introduced in 1959.
Extraction and presentation
Polychlorkupferphthalocyanin is by chlorination of copper phthalocyanine in an aluminum chloride - / sodium chloride - melt obtained, whereby to date than 10,000 t of which annually produced more.
properties
As with phthalocyanine blue, the central atom of the ring-shaped halogenated compound is a Cu atom. This ensures increased color stability , which is additionally increased by the chlorination. In addition, there is the generally high level of fastness of polycyclic pigments. In addition, this pigment is non-toxic and extremely resistant to solvents , acids and alkalis .
use
In industrial applications in paints , plastics and high-quality printing inks, phthalocyanine green is the standard green pigment used due to its excellent level of fastness.
For use in paints and printing inks, phthalocyanine green is available in stores under various names (real green, helio real green, pigment green 7, Vert héliogène, monastral green). Since the pigment is non-toxic and has a strong color, it is used for soaps such as toilet soap, syndet soap and liquid soap. It is used as an additive in shampoos, shower baths, dishwashing detergents, laundry detergents and fabric softeners. It is used as a light-controlling additive in contact lenses . As a school paint, the pigment is known as dark green .
Yellow types
If the 3-position on all four benzene rings is brominated instead of chlorinated, the 2Y type of phthalocyanine green is formed. If all chlorine atoms have been replaced, it is the 8Y type. All yellow-green brominated pigments are summarized in the Color Index as CI Pigment Green 36. The color of these pigments is significantly more yellowish and the higher the number of bromine atoms in the molecule, the color becomes significantly more yellow. The properties of pigments from different manufacturers differ slightly. All of these pigments are green powders with a density of 2.3 to 2.7 g / cm³. The oil requirement is 23 to 28% and is therefore lower than with CI Pigment Green 7. Color purity, color strength and opacity also correspond to the properties of the unbrominated green pigment, but the production costs of the yellow-green pigments are higher. The commercial price of the yellow types is almost a third higher, so the production quantities are only a quarter of the amount of the chlorinated pigment. The two pigments differ significantly in their IR spectra.
Individual evidence
- ↑ a b c Entry on polychloro copper phthalocyanine in the GESTIS substance database of the IFA , accessed on December 19, 2019 (JavaScript required)
- ↑ Entry on phthalocyanine green at ChemBlink , accessed on February 25, 2011.
- ↑ a b Toxicological assessment of copper phthalocyanine, chlorinated (PDF) at the professional association for raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ W. Herbst, K. Hunger: Industrial organic pigments . 3rd edition, Wiley-VCH, Weinheim 2004.
- ^ Temple C. Patton: Pigment Handbook 1-Do-2 . Vol. I, John Wiley & Sons.
