Copper phthalocyanine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
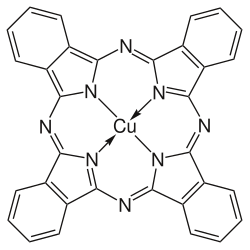
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Copper phthalocyanine | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 32 H 16 CuN 8 | |||||||||||||||
| Brief description |
blue, odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 576.07 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.62 g cm −3 |
|||||||||||||||
| Melting point |
> 150 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Copper phthalocyanine is a chemical complex compound between copper and phthalocyanine . It is a blue solid that is in the form of a powder or needles with a metallic sheen. Copper phthalocyanine is the main blue pigment . It is used in many different ways for paints , plastics and printing inks . Copper phthalocyanine pigments have a high temperature resistance and excellent fastness properties to light, weather and chemical influences.
history
In 1927 Henri de Diesbach and E. von der Weid reported on the synthesis of copper phthalocyanine and its properties and color brilliance in the journal Helvetica Chimica Acta , but without recognizing its economic importance. 1934 produced ICI in Trafford Park ( Manchester ), the first copper phthalocyanine (CuPc) and brought it to market in Germany known as the Monastralechtblau B . In 1936 Bayer with its own manufacturing process of CuPc under the trade name Heliogenblau B thereto. The previously used inorganic pigments ultramarine and Prussian blue were largely replaced in the following years.
Extraction and presentation
Copper phthalocyanine is produced industrially by the reaction of phthalic anhydride with copper (I) chloride and urea and ammonium heptamolybdate as a catalyst with heating. An alternative process is the thermal reaction of phthalic acid dinitrile with metallic copper or copper salts in the presence of ammonia or urea.
In 1993 more than 10,000 tons were already being produced per year. This makes it one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible dangers (" Screening Information Dataset ", SIDS).
properties
Copper phthalocyanine exists in eleven modifications , three of which are of economic importance. These are the thermally less stable red-tinged α-form (CI Pigment Blue 15: 0, 15: 1 and 15: 2), the stable green-tinged β-form (CI Pigment Blue 15: 3 and 15: 4), and the strong reddish ε-form (CI Pigment Blue 15: 6).
use
The majority of copper phthalocyanine is used as a high-speed pigment. It is the most abundant pigment for the blue color range in varnishes and paints, plastics and printing inks. The blue recycling bins for paper waste are colored with this pigment. Various, particularly quality-monitored brands are approved as colorants for consumer products, for use in care products, stamp inks, cosmetic articles such as toothpaste and for food packaging materials.
In the area of paints and varnishes, the α- and the β-modification are the standard pigments used for the blue area . The ε-modification has a greener tone, but appears more reddish in paints due to the more unstable pigment properties. Due to this color change, this is a specialty for automotive metallic paints.
In printing inks, the β-modification of copper phthalocyanine (pigment blue 15: 3) is the cyan standard for three- color printing and four-color printing.
Copper phthalocyanine is one of the most frequently used donor materials in organic solar cells , where it is also responsible for a large part of the absorption due to its blue color. In fact, the first organic solar cell was based on copper phthalocyanine.
The central atom of copper stabilizes the compound against reduction, so that the Cu dihydrophthalocyanine can only be obtained with a strong reducing agent and is immediately oxidized again to the blue pigment. In contrast, cobalt phthalocyanine forms a stable, colorless dihydro compound that is stable to moderate oxidation. This is used in document papers as a security feature against oxidative falsification . The slightly colored dihydro-nickel compound is already by air oxygen to a greenish blue pigment oxidized - in accordance with the position in the PSE of the eighth transition group: cobalt - nickel - copper.
Some of the raw copper phthalocyanine is chlorinated and used as phthalocyanine green (CI Pigment Green 7). It is the most commonly used pigment for the green color range. Since it is completely chlorinated, the name polychloropperphthalocyanine is also common. For use as a blue disperse dye with a reddish tinge and good fastness properties, quantities of the pigment are sulfonated or sulfochlorinated and thereby acquire the properties necessary for use in the textile industry. A certain amount of this product is used as a safety substance against adulteration with organic solvents due to its solubility.
The reversibly water-soluble CuPc derivative Alcian blue is used in biochemistry for coloring and marking.
Web links
- Heliogen blue, phthalocyanine blue PB 15 , Kremer-Pigmente.de
Individual evidence
- ↑ Entry on CI 74160 in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d Entry on copper phthalocyanine in the GESTIS substance database of the IFA , accessed on August 17, 2019(JavaScript required) .
- ↑ a b c d OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Copper, phthalocyaninato- , accessed on October 3, 2014.
- ↑ Entry on copper phthalocyanine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ P. Erk, H. Hengelsberg: Phthalocyanine Dyes and Pigments in Porphyrin Handbook 19 (2003), pp. 105-149, ISBN 0-12-393229-7 .
- ^ CI-74160 on Cosmeticanalysis.com. Retrieved April 11, 2018.
- ^ W. Herbst, K. Hunger, Industrial Organic Pigments, 3rd edition, Wiley-VCH, Weinheim 2004.
- ↑ Yu-Sheng Hsiao, Wha-Tzong Whang, Shich-Chang Suen, Jau-Ye Shiu and Chih-Ping Chen: Morphological control of CuPc and its application in organic solar cells. In: Nanotechnology 2008, 19, 415603 doi : 10.1088 / 0957-4484 / 19/41/415603
- ↑ CW Tang: Twolayer organic photovoltaic cell . In: Appl. Phys. Lett. tape 48 , no. 18 , 1985, pp. 183-185 , doi : 10.1063 / 1.96937 .
