Biliverdin reductase
| Biliverdin reductase | ||
|---|---|---|

|
||
| Surface model of the BVR-B with NADP and biliverdin as rods according to PDB 1HE2 | ||
| Mass / length primary structure | 296/206 amino acids | |
| Secondary to quaternary structure | Monomer | |
| Cofactor | Zn 2+ | |
| Identifier | ||
| Gene name (s) | BLVRA , BLVRB | |
| Enzyme classification | ||
| EC, category | 1.3.1.24 , oxidoreductase | |
| Response type | Redox reaction | |
| Substrate | Biliverdin + NADPH / H + | |
| Products | Bilirubin + NADP + | |
| Occurrence | ||
| Parent taxon | multicellular animals | |
The biliverdin reductase (BVR) is an enzyme of the heme cleardown in animals, it catalyzes the reduction of biliverdin to bilirubin . Two paralogous isoforms are known in humans : BVR-A is mainly found in the liver and BVR-B is only expressed in the embryo . It is believed that BVR-A has other functions in the nucleus because it is capable of autophosphorylation and binding to DNA. BVR-B can also reduce flavins .
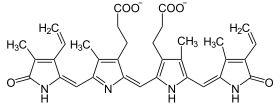
Catalyzed reaction
Biliverdin is reduced to bilirubin. BVR-A is the only known enzyme with a double activity profile depending on pH and cofactor (NADPH at pH 8.7 and NADH at pH 7.0).
Individual evidence
- ↑ InterPro entry
- ↑ UniProt P53004
- ↑ a b Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PE, Maines MD: Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin . In: Biochem. J. . 413, No. 3, August 2008, pp. 405-16. doi : 10.1042 / BJ20080018 . PMID 18412543 . PMC 2723824 (free full text).
Web links
Wikibooks: Biochemistry and Pathobiochemistry: Porphyrin Breakdown - Learning and Teaching Materials