Uroporphyrinogen III synthase
| Uroporphyrinogen III synthase | ||
|---|---|---|
|
||
| Uroporphyrinogen III synthase monomer, Thermus thermophilus | ||
| Properties of human protein | ||
| Mass / length primary structure | 265 amino acids | |
| Secondary to quaternary structure | Monomer | |
| Identifier | ||
| Gene name | UROS | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 4.2.1.75 , lyase | |
| Response type | Ring closure, rearrangement | |
| Substrate | Hydroxymethylbilane | |
| Products | Uroporphyrinogen III + H 2 O | |
| Occurrence | ||
| Parent taxon | Creature | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 7390 | 22276 |
| Ensemble | ENSG00000188690; | ENSMUSG00000030979 |
| UniProt | P10746 | P51163 |
| Refseq (mRNA) | NM_000375 | NM_001302085 |
| Refseq (protein) | NP_000366 | NP_001289014 |
| Gene locus | Chr 10: 125.78 - 125.82 Mb | Chr 7: 133.69 - 133.71 Mb |
| PubMed search | 7390 |
22276
|
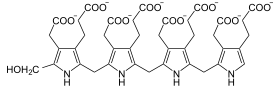
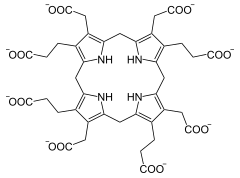
The uroporphyrinogen III synthase (URO3S) is the enzyme in animals that the ring closure of hydroxymethylbilane to Uroporphyrinogen III catalyzes a step in the part Porphyrinbiosynthese . URO3S is found in all tissue types in humans. Mutations in the UROS - gene can URO3S deficiency and this to Günther's disease lead.
Catalyzed reaction
Hydroxymethylbilane cyclizes to uroporphyrinogen III.
Individual evidence
Web links
Wikibooks: Biochemistry and Pathobiochemistry: Porphyrin Biosynthesis - Learning and Teaching Materials
- Jassal, D'Eustachio / reactome: Conversion of HMB to uroporphyrinogen III