Protoheme IX farnesyl transferase
| Protoheme IX farnesyl transferase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | <443 amino acids | |
| Identifier | ||
| Gene name | COX10 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 2.5.1. , Transferase | |
| Response type | Transfer of a farnesyl residue | |
| Substrate | Heme b | |
| Products | Haem o | |
| Occurrence | ||
| Homology family | Protoheme IX farnesyl transferase | |
| Parent taxon | Creature | |
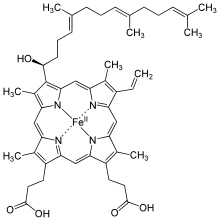
The protoheme IX-farnesyl transferase (COX10) (also: heme-o-synthase) is the enzyme in all living things, the conversion of b heme to heme o catalyzed . This is the first of three sub-steps on the metabolic pathway to the biosynthesis of heme a, which functions as the prosthetic group of cytochrome c oxidase . In eukaryotes , COX10 is found in the intermembrane space of the mitochondria . By mutations on COX10 - gene induced COX10 deficiency is the cause of rare inherited disorders such as cytochrome c oxidase deficiency or Leigh syndrome .
Together with the protein farnesyl transferase , COX10 belongs to the prenyl transferases .
Mice lacking the gene for COX10 showed fewer amyloid plaques in the brain in one study . The values for Abeta42 , beta-secretase and oxidative damage were also reduced. In a study with a "large number" of patients, a COX10 variant was associated with a reduced risk of Alzheimer's disease .
Catalyzed reaction
A farnesyl group is added to heme b, resulting in heme o.
Individual evidence
- ↑ UniProt Q12887
- ↑ Fukui H, Diaz F, Garcia S, Moraes CT: Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer's disease . In: Proc. Natl. Acad. Sci. USA . 104, No. 35, August 2007, pp. 14163-8. doi : 10.1073 / pnas.0705738104 . PMID 17715058 . PMC 1955773 (free full text).
- ↑ Vitali M, Venturelli E, Galimberti D, Benerini Gatta L, Scarpini E, Finazzi D: Analysis of the genes coding for subunit 10 and 15 of cytochrome c oxidase in Alzheimer's disease . In: J Neural Transm . October 2009. doi : 10.1007 / s00702-009-0324-8 . PMID 19826901 .