Methylglycinediacetic acid trisodium salt
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Simplified structural formula of the trinatium salt without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methylglycinediacetic acid trisodium salt | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 8 NNa 3 O 6 | ||||||||||||||||||
| Brief description |
yellowish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 271.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| pK s value |
|
||||||||||||||||||
| solubility |
590 g dm −3 (25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Methylglycinediacetic acid trisodium salt ( MGDA-Na 3 ) or trisodium-α- DL -alanine diacetate or α-ADA ), is based on the trianion of N - (1-carboxyethyl) iminodiacetic acid and is a tetradentate complexing agent , the stable 1: 1 chelate complexes with Cations with a charge number of at least +2, e.g. B. forms the "water hardness builders" Ca 2+ or Mg 2+ . In comparison to the isomeric β-alanine diacetic acid, α-ADA is more easily biodegradable and therefore more environmentally friendly.
presentation
The patent literature on industrial MGDA synthesis describes the approaches to solving the crucial requirements for a manufacturing process that can be implemented on an industrial scale, characterized by
- Achieving the highest possible space-time yields
- Simple reaction procedure at relatively low pressures and temperatures
- Realization of continuous process options
- Achieving low levels of impurities as possible, in particular with the on suspicion of carcinogenicity standing nitrilotriacetic
- Use of inexpensive raw materials, e.g. B. instead of pure L -alanine, the raw mixture of the Strecker synthesis of acetaldehyde , hydrogen cyanide and ammonia
- Avoidance of costly and yield-reducing isolation steps; instead, the resulting reaction solutions or precipitates are used directly in the subsequent process step
An obvious synthetic route to α-alanine diacetic acid starts from racemic α- DL -alanine, which is obtained by double cyanomethylation with formaldehyde and hydrogen cyanide , hydrolysis of the intermediate diacetonitrile to the trisodium salt and subsequent acidification with mineral acids in a yield of 97.4% d.Th. delivers over both stages.
In a later patent specification, with practically the same amounts of substance and under practically identical reaction conditions, only a total yield of 77% of theory is obtained. and an NTA content of 0.1%. This patent also specifies a process route via alaninonitrile, which is obtained by Strecker synthesis from hydrogen cyanide, ammonia and formaldehyde and converted to methylglycinonitrile- N , N- diacetonitrile by double cyanomethylation (step 1). The three nitrile groups are then hydrolyzed with sodium hydroxide solution to the trisodium salt of methylglycinediacetic acid (step 2).
The total yield is given as 72% of theory, the NTA content as 0.07%.
Another variant is via iminodiacetonitrile or iminodiacetic acid (step 1 '), which reacts in a weakly acidic medium (pH 6) with hydrocyanic acid and acetaldehyde to form methylglycinonitrile- N , N- diacetic acid, the nitrile group of which is hydrolyzed with sodium hydroxide solution to form MGDA-Na 3 ( Step 2'). The starting material iminodiacetic acid can be obtained inexpensively by dehydrating diethanolamine .
The total yield in this process variant is 72% of theory, the NTA content 0.07%.
Another variant is suitable for continuous process management, in which ammonia, formaldehyde and hydrogen cyanide react at pH 6 to form iminodiacetonitrile, which in a strongly acidic medium (pH 1.5) with acetaldehyde in a very good yield of 92% of theory. the trinitrile gives methylglycinonitrile- N , N -diacetonitrile (step 1).
Alkaline hydrolysis (step 2) leads to a total yield of 85% of theory. MGDA-Na 3 with an NTA content of 0.08%. This process variant seems to best meet the mentioned optimization criteria.
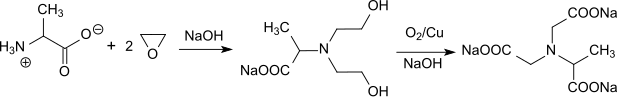
Recently, a low-by-product MGDA-Na 3 synthesis route was described in which alanine is ethoxylated with ethylene oxide in an autoclave to give bis-hydroxyethylaminoalanine and then oxidized at 190 ° C. with Raney copper under pressure to give α-ADA.
The yields are over 90% of theory, the NTA contents below 1%. The process conditions make this variant appear less attractive.
properties
The commercially available trisodium salt (84% by weight) of α- DL -alanine diacetic acid is a colorless, readily water-soluble solid, the aqueous solutions of which are quickly and completely broken down even by non-adapted bacteria. The aquatic toxicity towards fish, daphnia and algae is low. MGDA-Na 3 is described as easily biodegradable (OECD 301C) and> 90% is eliminated in sewage treatment plants. So far, α-ADA could not be detected in the process of municipal and industrial sewage treatment plants. In addition to their very good biodegradability, MGDA-Na 3 solutions are distinguished from other complexing agents of the aminopolycarboxylate type by high chemical stability even at temperatures above 200 ° C (under pressure) in a broad pH range between 2 and 14, as well as by high complex stabilities out.
The following table shows the complex formation constants log K of MGDA compared to tetrasodium iminodisuccinate IDS and ethylenediaminetetraacetic acid EDTA compared to polyvalent metal ions:
| Metal ions | MGDA | IDS | EDTA |
|---|---|---|---|
| Ba 2+ | 4.9 | 3.4 | 7.9 |
| Ag + | 3.9 | 7.3 | |
| Sr 2+ | 5.2 | 4.1 | |
| Ca 2+ | 7.0 | 5.2 | 10.6 |
| Mg 2+ | 5.8 | 6.1 | 8.7 |
| Mn 2+ | 8.4 | 7.7 | 13.8 |
| Fe 2+ | 8.1 | 8.2 | 14.3 |
| Cd 2+ | 10.6 | 8.4 | 16.5 |
| Cr 3+ | 9.6 | ||
| Co 2+ | 11.1 | 10.5 | 16.3 |
| Zn 2+ | 10.9 | 10.8 | 16.5 |
| Pb 2+ | 12.1 | 11.0 | 18.0 |
| Ni 2+ | 12.0 | 12.2 | 18.6 |
| Cu 2+ | 13.9 | 13.1 | 18.8 |
| Al 3+ | 14.1 | 16.1 | |
| Hg 2+ | 14.9 | 21.8 | |
| Fe 3+ | 16.5 | 15.2 | 25.1 |
The complex formation constants of the readily biodegradable chelators MGDA and IDS are in a range that is useful for industrial use, but well below those of the previous EDTA standard.
In solid preparations, α-ADA is stable to oxidizing agents such as perborates and percarbonates , but not to oxidizing acids or sodium hypochlorite .
use
Like other complexing agents from the aminopolycarboxylic acid class , α-alanine diacetic acid is found due to its ability to form stable chelate complexes with polyvalent ions, in particular the water hardness components Ca 2+ and Mg 2+ , as well as transition and heavy metal ions such as Fe 3+ , Mn 2+ , Cu 2+ etc. Use in water softening, in detergents and cleaning agents, in electroplating, cosmetics, paper and textile production. Because of its stability at high temperatures and pH values, MGDA-Na 3 should be particularly suitable as a replacement for the phosphates banned in the EU from 2017, such as sodium tripolyphosphate (in English STPP sodium tripolyphosphate ) in tabs for dishwasher machines.
BASF SE is the most important manufacturer of MGDA-Na 3 under the brand name Trilon M , has large-scale plants in Ludwigshafen and Lima, Ohio, and is currently expanding existing capacities with a further large-scale plant at the Evonik site in Theodore, Alabama.
Individual evidence
- ↑ a b c d e f g h i BASF SE, Technical Information : Trilon M Types
- ↑ National Industrial Chemicals Notification and Assessment Scheme (NICNAS): Full Public Report "Methyl glycine diacetic acid, trisodium salt" ( Memento of the original dated February 12, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this note. , File No: STD / 1092, August 2004.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b BASF, safety data sheet : Trilon M Powder
- ↑ Environmental Protection Agency , DfE's Safer Chemical Ingredients List, Chelating Agents , Alanine, N, N-bis (carboxymethyl) -, sodium salt (1: 3) .
- ↑ Patent WO9429421 : Use of glycine-N, N-diacetic acid derivatives as biodegradable complexing agents for alkaline earth metal ions and heavy metal ions, and methods of preparing them. Published on December 22, 1994 , Applicant: BASF AG, inventor J. Schneider et al ..
- ↑ a b Patent US5849950 : Preparation of glycine-N, N-diacetic acid derivatives. Published on December 15th, 1998 , Applicant: BASF AG, inventors T. Dalberg et al ..
- ↑ Patent EP2547648 : Process for the production of low-by-product aminocarboxylates. Published on January 23, 2013 , Applicant: BASF SE, Inventor: R. Baumann et al ..
- ↑ Hessian State Office for Environment and Geology, 6.12 Complexing Agents . 2003, p. 12/6.
- ↑ D. Kołodyńska, H. Hubicka, Z. Hubicki: Studies of application of monodisperse anion exchangers in sorption of heavy metal complexes with IDS . Desalination , 239, No. 1-3, pp. 216-228, doi : 10.1016 / j.desal.2008.02.024 .
- ^ Lanxess AG, General Product Information : Baypure
- ^ BASF SE, Technical Information : Trilon B Types
- ↑ SEPAWA, Review 2013, Abstracts: Detergents and Cleaning Agents Session Cleaning and Hygiene , Jürgen Kielholz: Phosphate-free detergents for automatic dishwashers ( Memento of the original from July 14, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ BASF SE: No more tea stains and chalky deposits