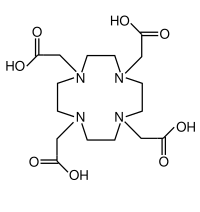
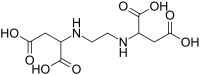
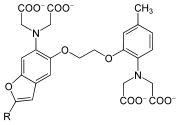
Aminopolycarboxylic acids
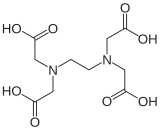
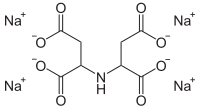
The aminopolycarboxylic acids or complexones are a group of complexing agents which consist of one or more nitrogen-containing groups and several carboxy groups . EDTA and NTA are used in the largest quantities. However, EDTA is not biodegradable and NTA is considered to be carcinogenic . Therefore, new chelators such as tetrasodium iminodisuccinate, methylglycinediacetic acid and β-alaninediacetic acid are gaining in importance.
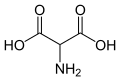
The natural amino acids aspartic acid and glutamic acid are aminodicarboxylic acids and are thus also aminopolycarboxylic acids.
They are needed for chelatometry and used in detergents .
Natural aminopolycarboxylic acids
Some living things use aminopolycarboxylic acids as siderophores . Bacteria ( S , S - enantiomer ) and fungi ( R , R -E.) Produce rhizoferrin and rhizobactins . In sweet grasses , oats use avenic acid and barley uses mugineic acid to absorb iron from the soil. Nicotianamine is present in practically all higher plants .
Some of these form significantly more stable complexes with iron than the synthetic aminopolycarboxylic acids.
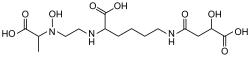
Synthetic aminopolycarboxylic acids
Individual evidence
- ↑ Aminopolycarboxylic acids ( Memento of the original dated August 24, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b Margarete Bucheli-Witschel, Thomas Egli: Environmental fate and microbial degradation of aminopolycarboxylic acids . In: FEMS Microbiology Reviews . tape 25 , no. 1 , January 2001, p. 69-106 , doi : 10.1111 / j.1574-6976.2001.tb00572.x .
- ↑ Tame Devil . In: Der Spiegel . No. 42 , 1971, p. 198 ( online ).
- ↑ Complexometric Titration with Aminopolycarboxylic Acids (EDTA and Analogs). Retrieved July 11, 2014 .
- ↑ Shinji Fushiya, Yoshikazu Sato, Shigeo Nozoe, Kyosuke Nomoto, Tsunematsu Takemoto, Sei-ichi Takagi: Avenic acid, a new amino acid possessing an iron chelating activity . In: Tetrahedron Letters . tape 21 , no. 32 , January 1980, p. 3071-3072 , doi : 10.1016 / s0040-4039 (00) 77410-7 .
- ↑ Mugineic acid, a phytosiderophores
- ↑ Waldemar Ternes: Biochemistry of the elements: Inorganic chemistry of biological processes . Springer DE, 2013, ISBN 3-8274-3020-8 , pp. 115 ( limited preview in Google Book search).
- ↑ Miloš Buděšínský, Herbert Budzikiewicz, Želimír Procházka, Helmut Ripperger, Axel Römer, Günter Scholz, Klaus Schreiber: Nicotianamine, a possible phytosiderophore of general occurrence . In: Phytochemistry . tape 19 , no. January 11 , 1980, p. 2295-2297 , doi : 10.1016 / s0031-9422 (00) 91014-8 .
- ↑ G. Schwarzenbach, E. Kampitsch, R. Steiner: Complexones II. The ability of iminodiacetic acid, methylimino-diacetic acid, aminomalonic acid and aminomalonic acid-diacetic acid to form complexes . In: Helvetica Chimica Acta . tape 28 , no. 1 , 1945, p. 1133–1143 , doi : 10.1002 / hlca.194502801158 ( PDF ).
- ^ A b c Giorgio Anderegg, Francoise Arnaud-Neu, Rita Delgado, Judith Felcman, Konstantin Popov: Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications * (IUPAC Technical Report). In: Pure and Applied Chemistry. 77, 2005, doi: 10.1351 / pac200577081445 . PDF