Wilkinson's catalyst
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Ph = phenyl | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Wilkinson's catalyst | ||||||||||||||||||
| other names |
Chloridotris (triphenylphosphine) rhodium (I) ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 54 H 45 ClP 3 Rh | ||||||||||||||||||
| Brief description |
dark red, odorless, solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 925.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
157 ° C |
||||||||||||||||||
| solubility |
bad in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
The Wilkinson's catalyst is in the organic chemistry used homogeneous catalyst with the empirical formula C 54 H 45 ClP 3 Rh. This is a rhodium complex , of the hydrogenation , hydroformylation , hydrosilation and isomerization of allyl groups to propenyl groups application finds. The Wilkinson catalyst is named after its developer, Nobel Prize winner Geoffrey Wilkinson .
Structure and synthesis
When Wilkinson's catalyst is a square planar rhodium (I) complex having a chloridogold - and three triphenylphosphane - ligand (PPh 3 carries). It is a 16- valence electron complex . It can be synthesized by substituting triphenylphosphine for rhodium (III) chloride in boiling ethanol . Since the reaction also takes place in mixtures of acetone and water instead of ethanol, but only in the presence of an excess of triphenylphosphine, it can be assumed that the latter acts not only as a substituent but also as a reducing agent (reduction of Rh (III) to (Rh ( I)).
Catalytic cycle
The Wilkinson hydrogenation is used for the hydrogenation of alkenes with molecular hydrogen . The decisive factor here is the lability of the bound phosphine ligands, the splitting off of which creates free coordination sites. In the first step, a phosphine ligand splits off from the catalyst. Then hydrogen adds oxidatively to the previously formed trigonal-planar 14-valence electron species. A trigonal-bipyramidal complex is formed here. The oxidation state changes from + I to + III. The alkene used then initially coordinates side-on on the metal. The alkene is then inserted with hydrogenation. A trigonal bipyramidal complex is formed again, which now bears an end-on- bonded alkyl radical . The hydrogenation by the second bonded hydrogen ultimately leads to splitting off ( reductive elimination ) of the alkane with regression of the catalyst species.
The Wilkinson catalyst can selectively hydrogenate terminal double bonds . The reaction takes place so much faster on these that another non-terminal double bond in the molecule is not attacked. In the case of sterically demanding substituents on the double bond and in the case of tetrasubstituted double bonds, there is usually no hydrogenation at all.
Asymmetric hydrogenations
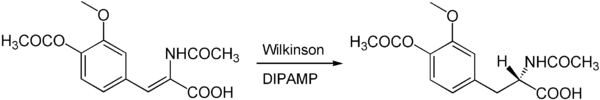
The Wilkinson catalyst can also be used for the asymmetric synthesis of chiral products. For this purpose, chiral phosphines such as DIPAMP or DIOP are used instead of the achiral triphenylphosphine ligands . For example, the center of chirality of the medically important amino acid L- DOPA can be built up via an asymmetric Wilkinson hydrogenation with DIPAMP as the chiral ligand.
Web links
Individual evidence
- ↑ a b c Entry for CAS no. 14694-95-2 in the GESTIS substance database of the IFA , accessed on December 2, 2007(JavaScript required) .
- ↑ Data sheet Tris (triphenylphosphine) rhodium (I) chloride from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ↑ JA Osborn, FH Jardine, JF Young, G. Wilkinson, Journal of the Chemical Society A . 1966, pp. 1711-1732.
- ↑ Beyer / Walter: Textbook of Organic Chemistry . Hirzel Verlag, 23rd edition. 1998, pp. 406f.
- ↑ Christen and Fritz Vögtle : Organische Chemie Vol. 2 , Otto Salle Verlag, 2nd edition, 1996, p. 411.
![{\ displaystyle \ mathrm {[RhCl_ {3} (H_ {2} O) _ {3}] + 4 \ PPh_ {3} \ longrightarrow \ [RhCl {(PPh_ {3})} _ {3}] + OPPh_ {3} +2 \ HCl + 2 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b0260e6848060515945d7c9099d18f7a61342b30)