Manganocene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Manganocene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 10 Mn | |||||||||||||||
| Brief description |
brown crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 185.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
175 ° C |
|||||||||||||||
| boiling point |
245 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Manganocene is an organometallic compound of manganese and is one of the metallocenes . The brown solid, pink above 158 ° C, has unusual magnetic properties.
Extraction and presentation
Like other metallocenes, manganocene can be produced from manganese (II) chloride and a cyclopentadienyl compound , usually cyclopentadienyl sodium . To this end, cyclopentadiene is first reacted with sodium and deprotonated.
Manganocene is then formed by reaction of sodium cyclopentadienyl with manganese (II) chloride in solvents such as tetrahydrofuran , ethylene glycol dimethyl ether or liquid ammonia .
properties
Since manganese has one less valence electron than iron, manganocene also has one less electron than ferrocene and does not meet the 18-electron rule with 17 electrons . Since the manganese is in a very favorable high-spin d5 configuration (every d orbital occupied by an electron), it cannot be reduced to Mn 1+ in order to obtain the favorable 18 electrons.
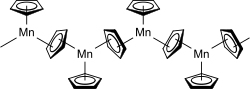
In the solid state manganocene lies in a polymeric prior structure in which each of three manganese cyclopentadienyl - ligand is surrounded. Two of the three ligands are connected to two manganese centers each, while the third is only adjacent to one manganese atom. A long chain is formed. The distances between the carbon atoms and the manganese are 242 pm for the terminal ligands and 240–330 pm for the bridged ligands. The bridging cyclopentadienyl ligands are not symmetrical between the manganese atoms.
Below the Néel temperature of 134 ° C manganocene is antiferromagnetically . Above this, the complex has a strong magnetic moment with a value of 5.81 Bohr magnetons .
Individual evidence
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 452 ( limited preview in the Google book search).
- ↑ a b c d data sheet bis (cyclopentadienyl) manganese, sublimed at AlfaAesar, accessed on December 1, 2019 ( PDF )(JavaScript required) .
- ↑ a b Christoph Elschenbroich : Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 451 ( limited preview in the Google book search).
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 468 ( limited preview in the Google book search).
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 460 ( limited preview in the Google book search).
- ↑ Erwin Riedel, Ralf Alsfasser, Christoph Janiak and Thomas M. Klapötke: Modern Inorganic Chemistry . 4th edition, von Gruyter, 2007, ISBN 978-3-11-019060-1 , p. 707.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1619.
- ↑ Christoph Elschenbroich: Organometallchemie . 6th edition, Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 455 ( limited preview in the Google book search).


