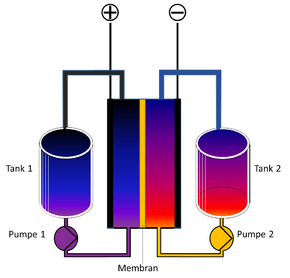
Redox flow battery
The redox flow battery (RFB) or ( redox ) flow battery - also known more generally as a liquid battery or wet cell - is an embodiment of an accumulator . It stores electrical energy in chemical compounds, with the reactants in a solvent in dissolved form. The two energy-storing electrolytes circulate in two separate circuits, between which the ion exchange takes place in the galvanic cell by means of a membrane . The dissolved substances are chemically reduced or oxidized in the cell , releasing electrical energy.
General
Compared to an accumulator without mass transfer, the construction is more complex, which in addition to the tank and pipelines requires at least two pumps for circulating the electrolytes, including the necessary control and monitoring devices. Therefore, flow batteries are not so good for small consumers. The use in the field of electromobility is the subject of research. The most frequently used and most important type of flow battery to date is the vanadium redox accumulator . In addition, there are other types such as the polysulphide -bromide accumulator , sodium chloride-redox accumulator , zinc-bromine accumulator and uranium-redox accumulator .
Since the chemical compounds dissolved in a solvent are stored in tanks that are separate from the cell and of any size, the amount of energy stored does not depend on the cell size. The redox flow battery is related to the accumulator and the reversible fuel cell due to its electrochemical reversibility . The cell voltage is given by the Nernst equation and in practically realizable systems is 1 V to 2.2 V.
research
history
The basics for redox flow cells were developed in Germany by Walther Kangro at the Technical University of Braunschweig in the middle of the 20th century , when the possibilities of energy storage with redox pairs were first examined. In the 1970s, NASA was busy developing the technology. The pure vanadium solution was first proposed in 1978 and developed in the 1980s at the University of New South Wales by Maria Skyllas-Kazacos and her colleagues. This solution was patented in 1986 and is the most common so far. It underwent a further development to the vanadium-bromide-based cell, which allows twice as high energy densities.
Current research
In January 2014, researchers at Harvard University presented a redox flow cell based on organic quinones that does not require the use of rare and therefore comparatively expensive substances. Power densities of 600 milliwatts per square centimeter were measured in prototypes. The long-term stability of such cells is currently being researched.
In 2015, a redox flow concept based on iron and zinc was presented in the journal Energy and Environmental Science . By using the cheap materials iron and zinc, a system price of less than 100 US dollars capital costs / kWh should be possible in the medium term, which would make these accumulators competitive with pumped storage and compressed air storage power plants . So far, redox flow cells have been around 300 to 800 US dollars / kWh. The power density would be around 680 milliwatts per square centimeter.
In 2015, the Friedrich Schiller University in Jena presented another innovation. A polymer- based redox flow battery (pRFB) that completely dispenses with metals as active material. This new type of battery uses organic polymers (similar to Plexiglas or Styrofoam), which carry a redox-active unit, for the anode and cathode. No corrosive acids are required as solvents, a simple saline solution is sufficient. The use of the aqueous polymer solutions also enables the use of dialysis membranes to separate the anode and cathode, which are much easier and cheaper to manufacture than classic ion exchange membranes. While the large macromolecules (polymers) are retained on the principle of a “sieve”, the small salt ions can pass through the membrane unhindered and close the cell-internal circuit.
Current research focuses on a. on lignin as a raw material. Due to its chemical properties in combination with its environmental friendliness, its wide availability and its low costs, lignin is considered to be a promising raw material for metal-free redox flow batteries for energy storage , especially for stationary storage of electricity from renewable energies . It is considered possible that flow cells based on lignin will in future have storage costs of approx. 3 ct / kWh with a storage efficiency of 90%.
technology
construction
The energy-storing electrolytes are stored outside the cell in separate tanks. This means that the redox flow cell with the tanks for the electrolytes - like the fuel cell with the tanks for the fuel and the oxidizing agent - is an electrochemical energy storage device in which the amount of energy and power can be scaled independently of one another. The tanks could be filled manually and the accumulator charged by metabolism. By exchanging the electrolyte fluids, they can also be charged and discharged in spatially separate accumulators; thus not the entire accumulator with converter technology and housing needs to be exchanged, but only the actual energy carrier between the charging and discharging stations. In practice, however, the systems are designed with circuits that are as closed as possible.
The actual galvanic cell is divided into two half-cells by a membrane. The electrolyte flows past the membrane. The half-cell is delimited by an electrode on which the actual chemical reaction takes place in the form of a reduction or oxidation. Depending on the cell type, the membrane is a microporous separator that allows all ions to pass through, or a selective anion or cation exchange membrane, or a size exclusion membrane that retains polymer and allows small ions to pass through. The membrane should prevent the two electrolytes from mixing.
Due to their high electrochemical voltage window in aqueous solutions, the electrodes are usually made of graphite . For the highest possible specific power, graphite felts with a high specific surface are used as electrode material.
electrolyte
The electrolyte consists of a salt dissolved in a solvent . The composition of the electrolyte, more precisely the concentration , largely determines the energy density of the redox flow battery with the cell voltage . Either inorganic or organic acids are often used as solvents. In newer systems, such as the polymer-based battery, simple saline solutions can also be used. Redox pairs that can be used include vanadium (V) oxide (in a vanadium redox accumulator) or sodium bromide (in a sodium bromide redox accumulator) in combination with other chemical compounds. Compounds based on organic substances such as lignin or lignin sulfonate solutions are also possible.
properties
Depending on the size and type, the redox flow cell can provide power from a few 100 watts to several megawatts and has an efficiency in the range of 75 to 80 percent. In addition, the system has a low self-discharge and a long service life . The latter is based on the fact that the electrode material itself does not react chemically during the reaction of the electrolyte and therefore does not degenerate. In contrast, the energy density is comparatively low; Usually about 25 Wh per liter of electrolyte liquid can be achieved with vanadium redox accumulators based on sulfate , and about 50 Wh per liter of electrolyte liquid based on bromide . Slightly higher values can also be achieved under ideal laboratory conditions.
With approx. 10 kWh per liter, diesel fuel has an energy density of approx. 400 times that of the electrolyte in vanadium redox batteries; commercially available lead-acid batteries achieve an energy density of around 42 Wh / kg, based on the total mass of the battery. In relation to the electrolyte of the lead accumulator, which makes up approx. 50% of the accumulator, the result is a value of around 80 Wh per liter of electrolyte liquid for a lead accumulator. Compared to a pumped storage plant with an energy density of 0.272 Wh / (l 100m) standardized to a height difference of 100 m, the energy density is, however, significantly higher.
The following table shows some redox flow battery types with the cell voltage and the energy density per liter of electrolyte liquid:
| Type | Cell voltage (V) |
Energy density per liter of electrolyte liquid (Wh / l) |
|---|---|---|
| Vanadium redox accumulator | 1.25 | 15-25 |
| Polysulphide bromide accumulator | 1.54 | 25-50 |
| Zinc-bromine accumulator | 1.85 | 50-80 |
Applications
Due to its properties, the redox flow cell is primarily used for tests and prototypes. Redox flow cells, for example in the form of vanadium redox accumulators, are used as a reserve source for cell phone base stations or buffer batteries for wind turbines .
A system of this type with an output of 4 MW and a storage capacity of 6 MWh is used in a Japanese wind turbine.
The redox flow battery of the Pellworm hybrid power plant has a storage capacity of 1.6 MWh and a charging / discharging capacity of 200 kW.
In a practical research project ( application center ), the Fraunhofer ICT in Pfinztal has been researching the buffering of strongly fluctuating wind energy of a 2 MW wind turbine with a 20 MWh redox flow battery since the end of September 2018 . In addition, a 500 kW solar system will be installed by 2021. The aim is to achieve a continuous power supply for electricity consumers during the main demand times.
For modern context-oriented experimental chemistry lessons , model experiments that can be used in schools have already been developed.
Web links
- Energy 2.0 technical report ( Memento from February 21, 2013 in the web archive archive.today )
- RWTH Aachen University, ISEA - Redox Flow Battery Systems ( Memento from August 13, 2011 in the Internet Archive )
- Redox flow batteries at the Fraunhofer Society
- Redox Flow Cell Development and Demonstration Project (PDF), NASA, 1977
- A gigantic battery underground. Spectrum of Science , September 24, 2018.
Individual evidence
- ↑ Yoshinobu Shiokawa, Hajimu Yamana, Hirotake Moriyama: An Application of Actinide Elements for a Redox Flow Battery . Ed .: Journal of Nuclear Science and Technology. tape 37 , no. 3 , 2000, pp. 253-256 , doi : 10.1080 / 18811248.2000.9714891 .
- ↑ Patent DE914264 : Method for storing electrical energy. Registered June 28, 1949 , published June 28, 1954 , applicant: Dr. Walther Kangro, Braunschweig, inventor: Dr. Walther Kangro, Braunschweig.
- ↑ Heinz Pieper: On the question of storing electrical energy in liquids . In: Dissertation Technical University of Braunschweig . Braunschweig 1958, OCLC 64523955 .
- ↑ W. Kangro, H. Pieper: On the question of the storage of electrical energy in liquids . In: Electrochimica Acta . tape 7 , no. 4 , 1962, pp. 435 to 448 , doi : 10.1016 / 0013-4686 (62) 80032-2 .
- ↑ Patent DE1006479 : Method for storing electrical energy in liquids. Applied on July 14, 1954 , published on April 18, 1957 , applicant: Dr. Walther Kangro, Braunschweig, inventor: Dr. Walther Kangro, Braunschweig (describes the use of multiple electrodes in which different parts are used for charging than for discharging. The patent is not very important for the further development of redox flow cells, but proves that Kangro is striving for further development Has.).
- ↑ Patent US3996064 : Energy storage system. Applied on August 22, 1975 , published on December 7, 1976 , assignee: NASA, inventor: Lawrence H. Thaller.
- ↑ Organic mega flow battery promises breakthrough for renewable energy. Harvard University website . Retrieved January 10, 2014.
- ↑ Brian Huskinson et al. : A metal-free organic –– inorganic aqueous flow battery. In: Nature . 505, (2014), 195–198, doi: 10.1038 / nature12909 .
- ↑ Gong et al., A zinc-iron redox-flow battery under $ 100 per kW h of system capital cost. In: Energy and Environmental Science . 8, (2015), 2941-2945, doi: 10.1039 / c5ee02315g .
- ↑ Tobias Janoschka, Norbert Martin, Udo Martin, Christian Friebe, Sabine Morgenstern, Hannes Hiller, Martin D. Hager, Ulrich S. Schubert : An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. In: Nature . (2015), doi: 10.1038 / nature15746 .
- ↑ Alolika Mukhopadhyay et al .: Metal-Free Aqueous Flow Battery with Novel Ultrafiltered lignin as Electrolyte . In: ACS Sustainable Chemistry & Engineering . tape 6 , no. 4 , 2018, p. 5394-5400 , doi : 10.1021 / acssuschemeng.8b00221 .
- ↑ Where can the green energy be parked? . In: Frankfurter Allgemeine Zeitung , May 21, 2019. Accessed May 21, 2019.
- ↑ LF Arenas, C. Ponce de León, FC Walsh: Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage . In: Journal of Energy Storage . tape June 11 , 2017, p. 119–153 , doi : 10.1016 / j.est.2017.02.007 ( sciencedirect.com [accessed June 2, 2017]).
- ↑ Katja Maria Engel: A gigantic battery in the underground. Spectrum of Science , September 24, 2018, accessed February 2, 2019 .
- ↑ Organic electrolytes - nature as a model. Retrieved February 18, 2019 .
- ↑ Redox flow batteries. In: carmen-ev.de. Retrieved July 27, 2014 .
- ↑ PS and PSG General Purpose Battery Specifications. Retrieved January 1, 2014 .
- ↑ Feasibility Study of Energy Storage Systems in Wind / Diesel Applications Using the HOMER Model Section 1 Introduction. (PDF).
- ↑ Power storage urgently needed - Fraunhofer Institute is upgrading .
- ↑ Energy in the tank. In: Fraunhofer Society . December 20, 2018, accessed October 12, 2019 .
- ↑ Dominique Rosenberg, Markus Behnisch, Svenja Pansegrau, Maike Busker, Walter Jansen: Storage of electrical energy with novel, organic batteries . In: Practice of Natural Sciences - Chemistry in School: PdN . tape 65 , no. 4 . Aulis-Verlag, 2016, ISSN 0177-9516 , p. 36–42 ( pedocs.de [PDF; accessed November 2, 2019]).