Alkaline manganese cell
The alkali-manganese cell , also known colloquially as the alkali-manganese battery or alkaline , sometimes more precisely referred to as the zinc-manganese oxide cell , is a galvanic cell and is one of the most important electrochemical energy stores. It belongs to the family of zinc-manganese dioxide cells and represents a further development of the zinc-carbon cell . The main work on the development of the alkali-manganese cell was carried out at Union Carbide by Karl Kordesch and others in the late 1950s and in 1960 patented. In the years that followed, the alkali-manganese cell replaced the zinc-carbon cell in most applications because of its higher capacity , greater security against leakage, better resilience and longer shelf life.
The alkali-manganese cell is one of the primary elements, that is to say the non-rechargeable batteries , even though it is basically rechargeable to a limited extent. There are versions designed for recharging, the RAM cells ( Rechargeable Alkaline Manganese ), which are counted among the secondary elements ( accumulators ). However, these have not found widespread use.
General
The alkali-manganese cell works with potassium hydroxide , the aqueous solution of potassium hydroxide , as an alkaline electrolyte . The cathode (positive electrode) is on the outside and is a metal cup coated with manganese dioxide on the inside , the anode (negative electrode) in the middle of the cell consists of zinc powder .
As a matter of principle, when a battery is discharged, regardless of whether it is an alkali-manganese cell or a zinc-carbon cell, the anode is chemically decomposed. In the zinc-carbon cell, the anode (negative pole) is on the outside and forms the cell housing in the form of a zinc cup, which dissolves when discharging and can easily leak. The battery is running out. In the alkaline-manganese cell, the anode is located inside the cell and the cathode (the metal case of the battery) remains intact when discharged, which reduces the risk of leakage.
The different designs have a bursting membrane as a precaution against overpressure in the cells, which can occur in the event of an electrical short circuit and overheating. In the interior, pressed manganese dioxide (brownstone) forms the cathode, which is in electrical contact with the metal cell housing, in the sectional view as a black ring within the jacket. The metallic cell housing is not involved in the cell reaction. The anode, arranged centrally inside, consists of a paste made of zinc powder and potassium hydroxide , wrapped in ion-permeable filter paper. In the middle there is a metal pin that makes electrical contact with the base plate and forms the negative pole.
Until the 1990s, the zinc electrodes of alkali-manganese cells were amalgamated with up to 2% mercury to make them durable. The mercury is not directly involved in the chemical process of the battery, but serves as protection against undesired corrosion of the zinc, which is triggered by metallic impurities such as copper, nickel, iron and cobalt in connection with the alkaline electrolyte potassium hydroxide. With a higher purity of the zinc electrode, values around 99.99% pure zinc are common, zinc corrosion could be avoided and the shelf life could also be achieved without mercury. Commercially available alkali-manganese cells meet the RoHS guidelines .
The most important types of alkaline manganese cells are cylindrical round cells (for example LR6 = alkaline manganese AA or Mignon ) and button cells (for example LR44). Several individual cells can also be combined to form batteries (e.g. 6LR61 = alkaline-manganese 9-volt block of six cells). In 2004, around 800 million alkaline-manganese round cells and around 400 million alkaline-manganese button cells were placed on the market in Germany.
Electrochemistry

As with the zinc-carbon battery, the oxidation of zinc and the reduction of manganese dioxide provide the electrical energy. The electrons of the cell released during the oxidation migrate from the anode , which in this case of a battery is the negative electrode, with power output through the external circuit, to the cathode , which in this case is the positive electrode. To balance the charge, OH - ions migrate from the cathode to the anode through the electrolyte .
Anode reaction
During the discharge, metallic zinc (Zn) is oxidized on the anode. Two electrons are given off, the oxidation number of zinc is increased from ± 0 to + II. The reaction product depends on the conditions under which the oxidation occurs. At the beginning of the discharge, i.e. at a high OH - concentration, the tetrahydroxozincate ion (Zn (OH) 4 2− ), or zincate for short , is formed via various intermediate stages, which is readily soluble in the alkaline electrolyte .
When the electrolyte is oversaturated with zincate, zinc oxide (ZnO) begins to precipitate.
As the discharge progresses, i.e. with a lower OH - concentration, zinc hydroxide (Zn (OH) 2 ) is then formed. Zinc oxide (ZnO) is slowly produced from this with the release of water .
Cathodic reaction
The manganese dioxide used as the cathode material is usually an electrolyte manganese dioxide ( γ-MnO 2 ) with high electrochemical activity. During the discharge, manganese dioxide (MnO 2 ) is first reduced to manganese oxide hydroxide (MnOOH) in the cathode . This homogeneous solid phase reaction is called the first discharge stage.
An electron is taken up during the reaction, the oxidation number of manganese is reduced from + IV to + III and a proton (H + ) is built into the crystal lattice of manganese dioxide. This reaction is unusual because the product α-MnOOH ( groutite ) has the same crystal structure as the starting material γ-MnO 2 .
Under certain conditions, in the case of mild discharges, manganese oxide hydroxide (MnOOH) can be reduced even further in a slow reaction. This reaction is called the second stage of discharge.
This reaction is a heterogeneous reaction; the actual reduction takes place in solution . The Mn 3+ ions go into solution as a complex [Mn (OH) 4 ] - and are reduced to [Mn (OH) 4 ] 2− . The actual solid product Mn (OH) 2 then precipitates out of the saturated [Mn (OH) 4 ] 2− solution.
Redox reaction
If only the first discharge stage is taken into account, the following results for the overall reaction in the alkali-manganese cell:
As can be seen from the overall reaction equation, water is consumed during discharge, a used alkali-manganese cell is therefore "dry".
Side reaction
Zinc is thermodynamically unstable in a strongly alkaline solution. As can be seen from the electrochemical series , zinc (Zn) is therefore oxidized as a side reaction in the anode and water (H 2 O ) is reduced to gaseous hydrogen (H 2 ).
This reaction, known as “gassing”, takes place when non-discharged and partially discharged cells are stored. The reaction rate is relatively slow for high-purity zinc. However, even small amounts of impurities (e.g. heavy metals such as iron , copper , molybdenum and nickel ) can drastically increase gassing.
properties
tension
The nominal voltage of the alkali-manganese cell is 1.5 V. By connecting several cells in series, higher voltages can be achieved. The 3LR12 normal battery with three cells can achieve 4.5 V, the 4LR61 flat pack with four cells 6 V and the 6LR61 E-block with six cells 9 V.
The actual open circuit voltage of a fresh alkali-manganese cell is at 20 ° C in the range from 1.57 V to 1.63 V. It mainly depends on the activity of the manganese dioxide used and the zinc oxide content in the electrolyte solution. The (average) load voltage depends on the load, it is typically 1.15 V to 1.18 V when discharging at 0.4 C (NiMH: 1.22 V to 1.25 V). Usually 1.0 V is used as the final discharge voltage .
discharge
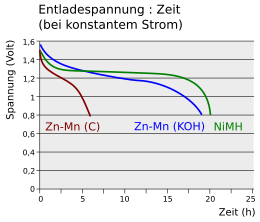
The diagram compares the load curves at constant current of a zinc-carbon cell (Zn-Mn (C)) and a NiMH battery with that of an alkali-manganese cell (Zn-Mn (KOH)). The voltage of the zinc-carbon cell drops below 0.8 V after a short time. A battery holds the voltage of 1.2 V over a long period of time. The time behavior of an alkali-manganese cell lies between the two curves, the voltage slowly decreases over time.
Battery level indicators in devices measure the change in voltage over time. While they work quite reliably with primary cells, they fail with NiMH cells. Here the voltage is almost constant over a long period of time, only to drop quickly when the battery is exhausted.
The capacity of an alkaline manganese cell depends on the load. With a low load << 0.1 C , AA batteries typically achieve values of 3000 mAh, see graphic on the left. The second graphic shows the course of the capacity with a load of approx. 0.1 C. The battery is already exhausted at 2/3 of the nominal capacity. In contrast to the behavior with a small load, it regenerates after a short time. It is again able to provide a capacity of approx. 10%, see lower curve in the second graphic.
Practice: Alkaline batteries in high-performance consumers seem to be empty after a short time. After a break of several hours, they work briefly again. A slightly higher temperature accelerates this regeneration. Hence the effect of “charging” batteries on the stove top or in the sun. However, these cells are then no longer able to meet the high demand for electricity. From the perspective of the high-performance consumer, the batteries are exhausted, although they may still have a remaining capacity of 30%. Instead of disposing of the cells, they should be used for consumers with lower power requirements, such as clocks or remote controls.
leak
In the case of alkaline batteries, the anode, which chemically decomposes during discharge, is inside the cell. This structurally prevents decomposition of the metallic outer shell, which is the cathode, in the case of the alkaline battery. However, alkaline batteries can also leak, for example due to overpressure inside as a result of a short circuit. As a result, the bursting membrane opens in the area of the negative connection and escaping potassium hydroxide can corrode metallic components such as contacts and conductor tracks. The potassium hydroxide reacts with the carbon dioxide (CO 2 ) in the air to form potassium carbonate (K 2 CO 3 ) and forms white, crystalline, hygroscopic deposits.
comparison
The following table contains a comparison between an alkali-manganese and a zinc-carbon cell in the Mignon design :
| Comparison of size Mignon | Alkali manganese | Zinc-carbon |
|---|---|---|
| Energy density | 350 Wh / dm³ | 150 Wh / dm³ |
| AA cell capacity (discharge up to 0.8 V) | 2.8 Ah | 1.2 Ah |
| Internal resistance | 0.15 Ω | 0.5 Ω |
| Self-discharge per month at 20 ° C | 0.3% | 0.6% |
| Remaining capacity after three years of storage |
> 90% | <10% |
| Minimum operating temperature | > −20 ° C | −10 ° C |
| Leak-proof | high | sufficient to bad |
| Costs per load removed | 8 ... 45 cents / ah | 17 ... 80 cents / ah |
Zinc-carbon cells have a significantly worse voltage level than alkali-manganese cells, i.e. H. the voltage of partially discharged cells drops early (see discharge ). The same applies to the high current capacity, which means that they can no longer be used for many modern devices that draw high currents for a short time (digital cameras, flash units, alarm systems). Even with fresh zinc-carbon cells, high current consumption causes the voltage to drop too low in a few seconds to ensure safe operation of the devices. The self-discharge of zinc-carbon battery cells is also significantly higher than that of alkali-manganese cells, which limits their shelf life.
Zinc-carbon batteries are also at a disadvantage in terms of economy and eco-balance , primarily due to the poorer nominal capacity with the same size and also lower usable capacity due to the poorer voltage level. This means that significantly more cells are required for the same energy expenditure (operating time of a device).
In addition, the leakage security is often worse. Leaking ammonium chloride solution from used cells destroys the metal contacts and conductor tracks of an electronic device much more than the alkaline electrolyte of the alkaline cells.
These clear differences have led to the fact that alkaline manganese cells dominate the market today and have displaced zinc-carbon cells.
Recharge
Normal alkaline manganese batteries
Most partially discharged alkaline manganese batteries (primary cells) can be refreshed three to ten times with suitable chargers (e.g. those for RAM cells; see the next section). The prerequisite is that the battery is not discharged too deeply. Chargers for commercially available NiMH batteries are not suitable for charging , as the threshold value for the end-of-charge voltage and the charging process do not match.
RAM cells
RAM cells ( English rechargeable alkali manganese ) are special alkaline manganese cells ( secondary cells) that can be recharged 50 to 500 times according to the provider . Commercially available chargers for RAM cells work with a constant charging current, which is interrupted every second for a few milliseconds in order to measure the cell voltage without current. If this exceeds 1.73 V, the charging current is switched off until the cell voltage has fallen below 1.69 V again. The constant voltage method is also safe and suitable, but slower. RAM cells are only suitable for low-power applications such as B. clocks or remote controls. For high current applications such as B. digital cameras, cordless tools or as drive batteries in model vehicles, they are not suitable and can be damaged.
In order not to lose their rechargeability, RAM cells must not be discharged too deeply. If RAM cells are discharged to a final discharge voltage of 1.42 V per cell, the achievable number of cycles is a few 100. With a discharge of up to 1.32 V, the number of cycles is reduced to a few 10. RAM cells cannot be discharged further more or only with a significantly reduced capacity.
As of October 2012, rechargeable RAM cells are the only batteries / accumulators that have been awarded the Blue Angel eco- label (label: “because rechargeable and low in pollutants”).
Price and performance
Commercially available alkaline manganese batteries are offered with large price differences. In a consumer test, a branded battery that was offered at six times the price of an inexpensive battery had a 25% longer service life than this.
disposal
In the EU, alkali-manganese batteries must be disposed of in accordance with the old battery directive (Directive 2006/66 / EC). This guideline is implemented differently in different countries, for example in Germany in the form of the Battery Act and in Austria in the form of the Battery Ordinance (BatterieVO). For practical implementation, the relevant trade is setting up collection containers from the Foundation for the Joint Take-Back System for Batteries .
literature
- Lucien F. Trueb, Paul Rüetschi: Batteries and accumulators - Mobile energy sources for today and tomorrow . Springer, Berlin 1998, ISBN 3-540-62997-1 .
- David Linden, Thomas B. Reddy (Eds.): Handbook of Batteries. 3. Edition. McGraw-Hill, New York 2002, ISBN 0-07-135978-8 (in English).
- Clive DS Tuck (Ed.): Modern Battery Technology . Ellis Horwood, New York 1991, ISBN 0-13-590266-5 (in English).
- Karl V. Kordesch (Ed.): Batteries Volume 1 Manganese Dioxide . Marcel Dekker, New York 1974, ISBN 0-8247-6084-0 (in English).
Web links
- Data sheets for some batteries from Energizer (in English)
Individual evidence
- ↑ Patent US2960558 : Dry cell. Applied on October 9, 1957 , published November 15, 1960 , Applicant: Union Carbide Corp. Inventors: Kordesch Karl, Paul A. Marsal, Lewis F. Urry.
- ↑ Dennis W. McComsey: Handbook Of Batteries . Ed .: David Linden, Thomas B. Reddy. 3. Edition. McGraw-Hill, 2002, ISBN 0-07-135978-8 , Chapter 8 .: Zinc-Carbon Batteries - Leclanché and Zinc Chloride Cell Systems.
- ↑ Foundation for a joint take-back system for batteries , success control 2004.
- ↑ AccuCell by Müller-Germany: Structure, comparison, advantages of RAM cells (PDF); accessed on August 8, 2012
- ↑ David Linden, Thomas Reddy (Ed.): Handbook of Batteries . 3. Edition. McGraw Hill, 2002, ISBN 0-07-135978-8 , Chapter 36: Rechargeable zinc / alkaline / manganese dioxide batteries .
- ↑ Federal Environment Agency (ed.): Advice on batteries and rechargeable batteries (PDF; 3.7 MB). Dessau, October 2012, p. 23.
- ↑ Aldi versus Lidl - The duel . ZDF documentary, first broadcast on April 2, 2013, 45 min.
- ↑ Directive 2006/66 / EC of the European Parliament and of the Council of September 6, 2006 on batteries and accumulators as well as used batteries and accumulators , accessed on September 23, 2017
- ↑ Batteries Regulation . (No longer available online.) Archived from the original on September 24, 2017 ; accessed on September 23, 2017 .


![\ mathrm {Zn + 4OH ^ - \ rightarrow [Zn (OH) _4] ^ {2-} + 2e ^ -}](https://wikimedia.org/api/rest_v1/media/math/render/svg/476efee5e57595690d0c0b4962f5f9baa7fc9945)
![\ mathrm {[Zn (OH) _4] ^ {2-} \ rightarrow ZnO + 2OH ^ - + H_2O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/daeb1985b7e1289e44ca69ac54d79654841147dd)




![\ mathrm {Zn + 2MnO_2 + 2H_2O + 2OH ^ - \ rightarrow [Zn (OH) _4] ^ {2-} + 2MnO (OH)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ab3b63d23ccd355114ddf07121236d59d58202d6)
![\ mathrm {Zn + 2H_2O + 2OH ^ - \ rightarrow [Zn (OH) _4] ^ {2-} + H_2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b04ee2db0f5da3e9f2dcc1fa46b66b77d81b7cf8)




