Vanadium redox accumulator

The vanadium redox accumulator (vanadium redox flow battery, VRFB) belongs - like all accumulators - to the class of rechargeable energy stores . It is a type of flow accumulator that uses vanadium compounds in aqueous solutions in both electrolytes . This avoids the problem of cross-contamination due to the diffusion of ions through the membrane.
Historical
The idea of using vanadium compounds in a battery for energy storage, is from 1933. 1976 patented a NASA -Staff first time the use of a vanadium salt in Redoxflusszellen, where he was then vanadium chloride suggested. The vanadium-vanadium pairing was filed for patent in 1978. Successful demonstration and commercial development did not take place until the 1980s at the University of New South Wales by Maria Skyllas-Kazacos and her staff. In 1998 the university sold the patents to an Australian company ( Pinnacle VRB ). After some restructuring and takeovers, the patents were finally taken over by Prudent Energy.
General
The vanadium redox battery in its current form with sulfuric acid - electrolyte was in 1986 by the University of New South Wales patented in Australia. The original patents expired in 2006, which enabled a free market and led to commercial developments.
The vanadium redox accumulator uses the ability of vanadium to be able to assume four different oxidation states in solution so that instead of two only one electroactive element is required for the accumulator. The nominal voltage per cell without load is in the range from 1.15 V to 1.55 V. At 25 ° C it is 1.41 V.
As with all flow batteries , a major advantage of the vanadium redox accumulator (VRFB) is that, unlike ordinary secondary cells, power and energy are independent of each other. Thanks to its modular structure, this enables the construction of a battery of any high power and capacity . The performance is v. a. through the electrode surface, the storage capacity can be regulated through the amount of electrolyte. Deep discharge is also problem-free. This means that the VRFB accumulator can be completely discharged for a long time without significant aging effects occurring. However, it has a comparatively low energy density of approx. 15 Wh / l to 25 Wh / l of electrolyte fluid. For comparison: conventional diesel fuel has an energy density of approx. 10 kWh / l that is approx. 400 times higher. The main disadvantage of vanadium redox accumulator technology, in addition to the poor volume-energy storage ratio, is the complex overall system in comparison to conventional accumulators, consisting of pumps and storage tanks. One of the most important advantages is the excellent stability over many charge-discharge cycles: A VRB battery in Japan has undergone more than 200,000 such cycles in a three-year test phase.
Reaction equations
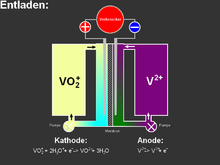
The vanadium redox accumulator uses redox pairs of vanadium in both half-cells . The solution on the positive side contains vanadyl sulfate (vanadine (IV) oxide sulfate, VOSO 4 , blue), which can be oxidized to the yellow pentavalent ion:
Positive electrode, positive pole, V (4+) and V (5+):
- VO 2+ + H 2 O ⇌ VO 2 + + 2 H + + e - ( E 0 = 0.995 V vs. SHE)
The solution on the negative pole side contains vanadium (III) sulfate (green), which can be reduced to the bivalent, purple vanadium salt:
Negative electrode, negative pole:
- V 3+ + e - ⇌ V 2+ ( E 0 = −0.255 V vs. SHE).
Side reactions
During charging - especially with high current densities - unwanted by-products can arise at the electrodes: Oxygen gas (O 2 ) on the positive pole side (anode ) or carbon dioxide through reaction with the carbon in the power supply. On the negative pole side (charging: cathode) hydrogen gas (H 2 ):
Positive electrode, positive pole: 6 H 2 O ⇌ O 2 + 4 H 3 O + + 4 e -
Positive electrode, positive pole: 6 H 2 O + C ⇌ CO 2 + 4 H 3 O + + 4 e -
Negative electrode, negative pole: 2 H 3 O + + 2 e - ⇌ H 2 + H 2 O
These reactions lower the efficiency of energy storage. The side reaction at the negative pole must also be avoided in order to prevent an accumulation of the flammable hydrogen gas.
Operational safety
Compared to other storage systems (especially lithium-ion batteries ), vanadium redox flow cells offer a very high level of operational reliability, since the electrolyte is neither flammable nor explosive due to its high water content. In a test, a VRFB survived an intentionally induced short circuit unscathed. Due to the separation between the performance-determining electrochemical cells and the storage tank, which determine the capacity, there is always only a small (percentage) part of the electrolyte in the converter unit (stack). Likewise, other aging and failure mechanisms typical for lithium-ion cells, such as the possible formation of dendrites and electrolyte deficiency and thermal runaway for aqueous redox flow cells, are also not relevant. To increase security with regard to possible risks from leaking electrolytes, there are z. B. Systems with a double-walled tank. Constant monitoring of the cell voltage and sensors for monitoring the electrolyte are also standard. When charging with high current densities, some hydrogen gas can be produced as a by-product due to water electrolysis, so that appropriate safety measures for current limitation and ventilation are taken.
Applications of vanadium redox flow accumulators

The currently available commercial batteries are only used stationary, e.g. B. in the areas of renewable energy sources to cover peak loads and as load balancing, also in the area of uninterruptible power supplies . As of May 2017, more than 40 large vanadium redox flow batteries are in operation worldwide. 10 of them have an output of 1 MW and more; 10 are in China, 5 in the US and 5 in Japan. Most large vanadium batteries are built near wind farms or large photovoltaic fields. The largest such battery is in Japan and has an output of up to 15 MW. Some vanadium redox flow systems are also in use in Germany, including three with outputs from 200 kW to 325 kW, as well as several systems of 10 kW (e.g. in the old country) or 20 kW (e.g. in Freiberg am Neckar). Many such smaller systems are operated worldwide, over 100 of which are from Gildemeister (January 2016, October 2015: 89, August 2014: 65).
The largest VRFB systems
Germany's largest vanadium flow battery, a flow cell system with a 660 m 3 tank capacity and 2 MW output and 20 MWh energy storage capacity, was completed in September 2019. The largest battery in the world will also be a vanadium redox flow cell battery. It should be able to generate 200 MW and store 800 MWh of energy. It will be installed in northeast China on the peninsula near Dalian and will consist of ten units with 20 MW and 80 MWh each. It is supplied by the industrial partners Rongke Power and UniEnergy Technologies (UET); the cost is $ 500 million.
| Battery storage plant | Location | power | energy | time | Installation
date |
operator | Manufacturer | Primary energy | supporting documents |
|---|---|---|---|---|---|---|---|---|---|
| Minami Hayakita |
|
15th | 60 | 4th | 01/06/2016 | Hokkaido Electric Power (HEPCO) | Sumitomo Electric Industries | Solar (111 MW) | |
| GuoDian LongYuan |
|
5 | 10 | 2 | 03/15/2013 | Longyuan Power | Rongke Power | Wind (Woniushi wind farm) | |
| Tomamae |
|
4th | 6th | 1.5 | 01/01/2005 | Hokkaido Electric Power (HEPCO) | Sumitomo Electric Industries | Wind 30.6 MW | |
| Sumitomo Densetsu |
|
3 | 0.8 | 0.27 | 02/01/2000 | Sumitomo Electric Industries | |||
| Zhangbei National V |
|
2 | 8th | 4th | December 01, 2011 | State Grid Corporation of China (SGCC) | Prudent Energy | Wind (100 MW) and solar (40 MW) | |
| Everett |
|
2 | 8th | 4th | 03/28/2017 | Snohomish County PUD | UniEnergy Technologies | ||
| San Diego |
|
2 | 8th | 4th | March 16, 2017 | San Diego Gas and Electric (SDG & E) | Sumitomo Electric (SEI) | ||
| Tottori Sanyo Electric |
|
1.5 | 1.5 | 1 | 04/01/2001 | Sumitomo Electric Industries | |||
| Yokohama |
|
1 | 5 | 5 | 07/24/2012 | Sumitomo Electric Industries | Solar 0.2 MW | ||
| Avista Pullman |
|
1 | 3.2 | 3.2 | 06/17/2015 | Avista | UniEnergy Technologies | ||
| Braderup |
|
0.325 | 1 | 3 | 09/15/2014 | Energy storage north | Vanadis Power (Rongke Power) | Wind (19.8 MW, community wind farm) | |
| Bielefeld |
|
0.26 | 0.65 | 2.5 | 09/15/2011 | Gildemeister Energy Solutions | Wind (1 MW) | ||
| Pellworm |
|
0.2 | 1.6 | 8th | 09/09/2013 | Gildemeister Energy Solutions | Wind 0.3 MW and solar 0.77 MW ( Pellworm hybrid power plant ) | ||
| RedoxWind Pfinztal |
|
2 | 20th | 10 | ??. 09.2019 | Fraunhofer Institute for Chemical Technology | Fraunhofer Institute for Chemical Technology | Wind (2 MW) |
Very limited suitability for use in vehicles
The weight-related energy density of the vanadium redox battery VRFB is higher than that of a lead-acid battery for batteries of 90 kW and more. In addition, they could theoretically be recharged quickly by exchanging the electrolytes, for example at special filling stations. Therefore, the VRFB was discussed for a while as an energy storage device for electric cars . In 1994 a golf course vehicle in Sydney was fitted with a VRFB. Light electric vehicles that were previously operated with lead batteries or undemanding electric vehicles can therefore be equipped with a VRFB. For powerful electric cars, however, the VRFB is not an option: The volumetric energy density of the VRFB is far too small, i. H. it takes up too much space. In addition, the VRFB is exceeded in many ways by today's lithium-ion batteries , which have undergone rapid development: the energy densities of lithium-ion batteries are significantly better in terms of both volume and weight, their energy efficiency is higher and they are maintenance-free.
Research and Development
Further research is currently being carried out on inexpensive membranes as an alternative to Nafion and highly concentrated electrolytes that are stable over wide temperature ranges. Catalysts are also being developed to increase the exchange current density and thus to increase efficiency.
Web links
- Side of the University of New South Wales for vanadium redox battery (Engl.)
- Research at the Fraunhofer Institute for Chemical Technology ICT
Individual evidence
- ↑ PA Pissoort, in French patent number 754065 of October 30, 1933
- ↑ Patent US3996064 : Electrically rechargeable REDOX flow cell. Filed August 22, 1975 , published December 7, 1976 , Applicant: National Aeronautics And Space Administration NASA, Inventor: Lawrence H. Thaller.
- ↑ Patent GB2030349 : Process and Accumulator, for Storing and Releasing Electrical Energy. Registered on July 10, 1978 , published on April 2, 1980 , applicant: Oronzio de Nora Impianti Elettrochimici SpA, inventor: Alberto Pellegri, Placido M. Spaziante.
- ↑ Patent DE2927868 : Method for storing and releasing electrical energy and a suitable accumulator. Published on January 31, 1980 , applicant: Oronzio de Nora Impianti Elettrochimici SpA, inventor: Alberto Pellegri, Placido M. Spaziante.
- ↑ M. Rychcik, M. Skyllas-Kazacos: Evaluation of electrode materials for vanadium redox cell . In: Journal of Power Sources . tape 19 , no. 1 , January 1987, pp. 45-54 , doi : 10.1016 / 0378-7753 (87) 80006-x ( elsevier.com ).
- ↑ M. Rychcik, M. Skyllas-Kazacos: Characteristics of a new all-vanadium redox flow battery . In: Journal of Power Sources . tape 22 , no. 1 , January 1988, p. 59-67 , doi : 10.1016 / 0378-7753 (88) 80005-3 ( elsevier.com ).
- ↑ M. Skyllas-Kazacos, M. Rychcik and R. Robins, in Australian patent number 575247 (1986), Unisearch Ltd.
- ↑ Spelters, Oliver (2010): Consideration of the dynamics of redox flow cells, Munich: Grin
- ↑ Redox flow batteries. Retrieved July 27, 2014 .
- ↑ Yitao Yan, Yifeng Li, Maria Skyllas-Kazacos, Jie Bao: Modeling and simulation of thermal behavior of vanadium redox flow battery . In: Journal of Power Sources . tape 322 . Elsevier BV, August 1, 2016, p. 116-128 , doi : 10.1016 / j.jpowsour.2016.05.011 ( sciencedirect.com ).
- ↑ Jens Tübke, Peter Fischer, Jens Noack: Redox flow batteries as stationary energy storage - status and perspectives. Retrieved May 26, 2017 .
- ^ AH Whitehead, TJ Rabbow, M. Trampert, P. Pokorny: Critical safety features of the vanadium redox flow battery . In: Journal of Power Sources . tape 351 . Elsevier BV, May 31, 2017, p. 1-7 , doi : 10.1016 / j.jpowsour.2017.03.075 ( sciencedirect.com ).
- ↑ Example ( Memento from April 23, 2016 in the Internet Archive ) (PDF) for a container-based system with a double-walled tank.
- ↑ M. Skyllas-Kazacos, MH Chakrabarti, SA Hajimolana, FS Mjalli, M. Saleem: Progress in Flow Battery Research and Development . In: Journal of The Electrochemical Society . tape 158 , no. 8 , August 1, 2011, ISSN 0013-4651 , p. R55 – R79 , doi : 10.1149 / 1.3599565 ( ecsdl.org [accessed May 28, 2017]).
- ↑ a b c DOE Global Energy Storage Database. In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, May 26, 2016, accessed on May 26, 2017 (English, This DOE database covers most major energy storage systems.)
- ↑ Press release from January 11, 2016 ( Memento from May 19, 2017 in the Internet Archive ) (PDF)
- ↑ Press release from October 20, 2015 ( Memento from May 19, 2017 in the Internet Archive ) (PDF)
- ↑ Press release of August 14, 2014 ( Memento of October 13, 2014 in the Internet Archive ) (PDF)
- ↑ a b Energy in the tank. Retrieved September 24, 2019 .
- ↑ a b The rotor is still standing still. Retrieved May 26, 2017 .
- ↑ a b major project »RedoxWind«. Fraunhofer Institute for Chemical Technology, accessed on May 26, 2017 .
- ↑ Press release on the planned largest battery in the world
- ↑ Junko Movellan: Hokkaido Is the New Solar Capital of Japan. In: Featured News. RenewableEnergyWorld.com, May 1, 2014, accessed May 26, 2017 .
- ↑ Minami Hayakita Substation Hokkaido Electric Power- Sumitomo. In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, September 20, 2016, archived from the original on November 9, 2017 ; accessed on May 26, 2017 (English).
- ↑ Kenji Kaneko: 60MWh Redox Flow Battery Starts Operations to Deal With Renewable Energy - News - Solar Power Plant Business. In: Solar Power Plant Business, News. Nikkei Business Publications, January 5, 2016, accessed May 26, 2017 .
- ↑ Project for wind farm energy storage Guodian Longyuan Woniushi. In: Market and Application, Integration of Renewable Energy. Rongke Power, accessed May 26, 2017 .
- ↑ Zonghao Liu, Huamin Zhang, Sujun Gao, Xiangkun Ma, Yufeng Liu: The world's largest all-vanadium redox flow battery energy storage system for a wind farm. Energy Storage Science and Technology, 2014, accessed May 26, 2017 .
- ↑ GuoDian LongYuan Wind Farm VFB. In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, May 26, 2016, accessed May 26, 2017 .
- ↑ Tomamae Wind Farm. In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, August 6, 2014, accessed May 26, 2017 .
- ^ Sumitomo Densetsu Office. In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, June 23, 2013, accessed May 26, 2017 .
- ↑ Clean Energy Ministerial - Energy Storage System: Challenges and Opportunities ( Memento of August 3, 2016 in the Internet Archive ), May 12, 2014 (PDF)
- ^ Zhangbei National Wind and Solar Energy Storage and Transmission Demonstration Project (V). In: DOE Global Energy Storage Database. Sandia Corporation, US Department of Energy, Strategen Consulting LLC, May 26, 2016, accessed May 26, 2017 .
- ^ A. Lee Barker: Sparton Resources Inc .: Special Shareholder Meeting Results November 3, 2016. In: News Room. Marketwired / Sparton Resources Inc., November 7, 2016, accessed May 26, 2017 .
- ↑ Megan Geuss: Washington state's new 8 megawatt-hour flow battery is the largest of its kind. In: Biz & IT. Ars Technica, WIRED Media Group, April 6, 2017, accessed on May 26, 2017 .
- ↑ Tom Kenning: SDG & E and Sumitomo unveil largest vanadium redox flow battery in the US. In: Energy Storage News. Solar Media Limited, March 17, 2017, accessed June 17, 2017 .
- ↑ Sumitomo Electric Starts Demonstration of Storage Battery System for Power Grid in California. In: Company Information> Press Release. Sumitomo Electric Industries, Ltd., March 17, 2017, accessed June 17, 2017 .
- ^ History of Vanadium Redox Battery. In: Vanadium Redox Battery. University of New South Wales, archived from the original on March 2017 ; accessed on June 4, 2017 .
- ↑ Vanadium Redox Flow Batteries. In: The Energy Blog. January 21, 2006, accessed June 4, 2017 .
- ↑ 4.1 Vanadium Redox Battery, Sumitomo Electric Industries Ltd. In: Review of Electrical Energy Storage Technologies and Systems and of their Potential for the UK. DTI Technology Program, Future Energy Solutions, EA Technolog, January 21, 2006, accessed June 4, 2017 .
- ↑ Demonstrating Megawatt-Class Power Generation / Storage System at Yokohama Works. In: Press Release> 2012. Sumitomo Electric Industries, Ltd., April 17, 2012, accessed on May 26, 2017 .
- ↑ Eric Wesoff, Jeff St. John: Largest Capacity Flow Battery in North America and EU Is On-Line and Commissioned. In: Biz & IT. Greentech Media, June 19, 2015, accessed May 26, 2017 .
- ↑ Vanadis Power supplies battery storage for hybrid systems . April 3, 2017 ( energieregion.de [accessed May 26, 2017]).
- ↑ IWR: Community wind farm uses Europe's largest megawatt hybrid battery . In: IWR . ( iwr.de [accessed on May 26, 2017]).
- ^ DOE Global Energy Storage Database. Retrieved May 26, 2017 .
- ^ The use of efficient storage systems on the Island of Pellworm. Archived from the original on May 15, 2017 ; accessed on May 26, 2017 (English).
- ↑ a b c d Álvaro Cunha, Jorge Martins, Nuno Rodrigues, FP Brito: Vanadium redox flow batteries: a technology review . Review. In: International Journal of Energy Research . tape 39 , no. 7 . John Wiley & Sons, June 10, 2015, ISSN 1099-114X , p. 889–918 , doi : 10.1002 / er.3260 (English).
- ^ Battery Pioneers: Maria Skyllas-Kazacos. Batteries International, September 22, 2016, accessed May 29, 2017 .
- ↑ Hongzhang Zhang, Huamin Zhang, Fengxiang Zhang, Xianfeng Li, Yun Li: Advanced charged membranes with highly symmetric spongy structures for vanadium flow battery application . In: The Royal Society of Chemistry (Ed.): Energy & Environmental Science . tape 6 , no. 3 , February 20, 2013, ISSN 1754-5706 , p. 776-781 , doi : 10.1039 / c3ee24174b ( rsc.org ).
- ↑ Liyu Li, Soowhan Kim, Wei Wang, M. Vijayakumar, Zimin Nie, B. Chen, J. Zhang, G. Xia, J. Hu, G. Graff, J. Liu, Zhenguo Yang: A Stable Vanadium Redox-Flow Battery with High Energy Density for Large-Scale Energy Storage . In: Advanced Energy Materials . tape 1 , no. 3 . Wiley-VCH, May 1, 2011, ISSN 1614-6840 , p. 394-400 , doi : 10.1002 / aenm.201100008 .