Cumene Hydroperoxide Process
The cumene hydroperoxide process is a chemical process for the production of phenol and acetone . It is also known as the Hock phenol synthesis or the Hock process and was named after its discoverer Heinrich Hock (1887–1971) who developed it in 1944.
Overview reaction
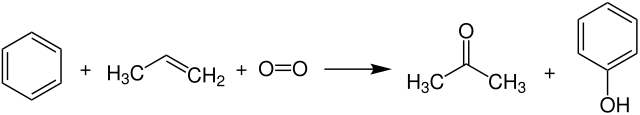
Benzene is alkylated by Friedel-Crafts alkylation with propene to give cumene , which is oxidized with oxygen to form a hydroperoxide and split with acids to give phenol and acetone.
The yield of this reaction for phenol is about 85 to 90% and for acetone about 60% of the amount of phenol.
Reaction mechanism
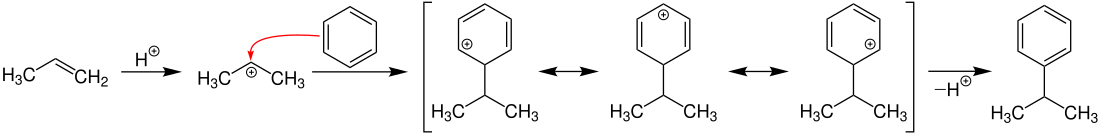
First there is an electrophilic aromatic substitution ( Friedel-Crafts alkylation ) of propene with benzene. By splitting off a proton, cumene (isopropylbenzene) is formed:
Chain start
To start the chain reaction, AIBN can be used, which generates a radical on cumene.
Chain propagation
The radical now present on cumene causes an attack on an oxygen atom, with another radical being created which attacks a cumene again. The resulting cumene radical can now react further.
Chain termination
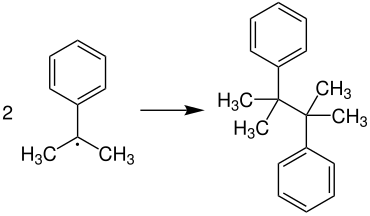
Since the radical does not always react with oxygen, chain breaks can occur. Two cumene radicals can dimerize, resulting in 2,3-dimethyl-2,3-diphenylbutane:
But it can also be that two cumene radicals disproportionate and thus cumene and 2-phenylpropene are formed:
The by-product 2-phenylpropene is recycled in production plants by hydrogenation with the formation of cumene.
Rearrangement
The cumene hydroperoxides formed in the chain reaction are split in acid by rearrangement into phenol and acetone. First there is a protonation of 1 , which creates 2 . This is followed by elimination of water and migration of the phenyl radical to the oxygen atom. This creates 3 (with the mesomeric boundary structures 3a and 3b ), which then reacts with water to form 4 . The splitting off of a proton leads to 5 . Its protonation at the other oxygen atom produces 6 - an oxonium ion - which ultimately breaks down to acetone and phenol with proton splitting.
Atomic economy
As long as there is demand for acetone in trade or industry, the process has excellent atomic economy, as virtually no waste is produced and the sulfuric acid can be reused. Since substances that are easy to manufacture are also used, this reaction is particularly important for industry.
application
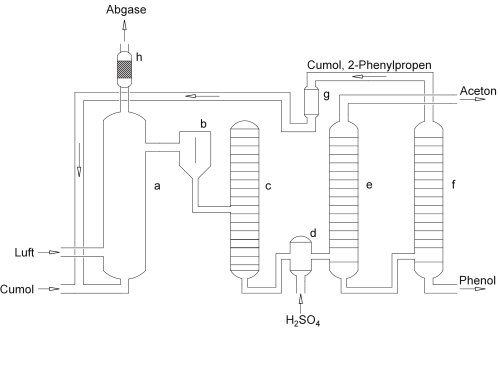
Compared to other phenol syntheses, the cumene hydroperoxide synthesis is characterized by the fact that only low raw material costs are incurred and only little energy is required. In the years following the discovery of this reaction, the proportion of phenol obtained through this reaction increased to 98.5% of the 8.7 million tons produced (2008).
Phenol is used in many ways. It finds uses for:
- The representation of aniline (by nucleophilic substitution ) for dyes, as well
- The production of bisphenol A for polymers.
- Phenol is also needed to make caprolactam , a substance used to make polyamides .
- The synthesis of phenoplasts , which are plastics.
variants
Instead of alkylating the benzene with propene, ethene can also be used. Phenol and acetaldehyde are then obtained as products .
Individual evidence
- ^ Heinrich Hock, Shon Lang: Autoxidation of hydrocarbons, IX. Mitteil .: About peroxides of benzene derivatives. In: Reports of the German Chemical Society . 77, 1944, p. 257, doi : 10.1002 / cber.19440770321 .
- ↑ a b Heinrich Hock, H. Kropf: Auto-oxidation of hydrocarbons and the cumene-phenol synthesis. In: Angewandte Chemie . 69, 1957, p. 313, doi : 10.1002 / ange.19570691002 .
- ↑ Manfred Weber, Markus Weber: phenol In: Phenolic Resins: A Century of Progress Springer, Berlin, Heidelberg 2010, pp 9-23, ISBN 978-3-642-04714-5 , doi : 10.1007 / 978-3-642 -04714-5_2 .
- ↑ Dr. Andrea Acker et al .: Hock method. In: Spektrum.de. Retrieved August 26, 2017 .