Lossen dismantling
The Lossen reaction (also Lossen degradation or Lossen rearrangement ) is a chemical reaction. Hydroxamic acid derivatives (usually O -acylated) are deprotonated on nitrogen and rearrange into an isocyanate with cleavage of the acyl radical. It is closely related to the Curtius degradation , the Hofmann degradation and the Schmidt reaction . It is mainly used to break down carboxylic acid chlorides to primary amines , but isocyanates or carbamates can also be obtained depending on the reaction conditions.
The reaction was named after its discoverer, the German chemist Wilhelm Lossen (1838–1906).
Reaction mechanism
Hydroxamic acid derivatives (= carboxylic acid hydroxylamides) are produced by the reaction of carboxylic acid chlorides with hydroxylamine.
The hydroxamic acid derivative reacts in the presence of a base (here: a hydroxide ion) and with the formation of water to form the corresponding salt. In the next step, an isocyanate is formed with splitting off of the carboxylate residue .
The reaction is described as concerted , but partly also with short-lived intermediate products ( nitrenes , electron-deficient compounds with an electron sextet on nitrogen). When carried out in an inert solvent, the isocyanates remain the end product of the reaction.
In the presence of water, the isocyanates formed react further to form the corresponding carbamic acid , which decarboxylates immediately and gives the primary amine as the end product . In the presence of alcohols , stable carbamic acid esters ( carbamates ) and amine- substituted ureas are formed .
Unsubstituted hydroxamic acids do not react, the hydroxyl group is too poor a leaving group. The substituent R, as a π or σ donor, can also facilitate the reaction. In hydroxamic acids with chiral radicals R *, their configuration is mostly retained.
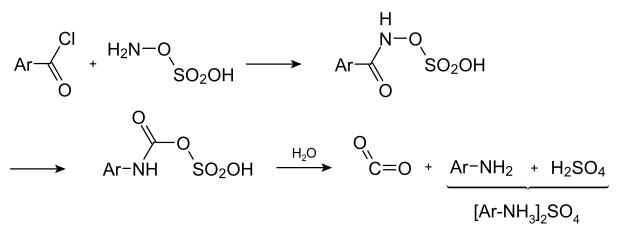
An interesting variant is the reaction of carboxylic acid chlorides with hydroxylamine- O- sulfonic acid : Here, the reaction product breaks down when heated to form amine, carbon dioxide and sulfuric acid.
literature
- T. Shiori: In: Comp. Org. Syn. 6, 1991, pp. 821-825 (review article).
- Hauser, CR; Renfrow, Jr., WB: Benzhydroxamic Acid In: Organic Syntheses . 19, 1939, p. 15, doi : 10.15227 / orgsyn.019.0015 ; Coll. Vol. 2, 1943, p. 67 ( PDF ).
- Thomas Laue, Andreas Plagens: Name and catchword reactions in organic chemistry . Vieweg + Teubner, Wiesbaden 2009, ISBN 978-3-8351-0091-6 , p. 218 ( limited preview in the Google book search).
Individual evidence
- ↑ W. Lossen: About benzoyl derivatives of hydroxylamine . In: Justus Liebig's Annals of Chemistry . tape 161 , no. 2-3 , 1872, pp. 347-362 , doi : 10.1002 / jlac.18721610219 .
- ↑ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press, 2005, ISBN 978-0-12-429785-2 , pp. 266-267.
- ^ Ludwig Bauer, Otto Exner: The Chemistry of Hydroxamic Acids and N-Hydroxyimides . In: Angewandte Chemie International Edition in English . tape 13 , no. 6 , June 1, 1974, pp. 376-384 , doi : 10.1002 / anie.197403761 (review article).
- ↑ Harry L. Yale: The Hydroxamic Acids . In: Chemical Reviews . tape 33 , no. 3 , December 1, 1943, p. 209-256 , doi : 10.1021 / cr60106a002 (review).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 81–90. Edition. Walter de Gruyter, Berlin 1976, ISBN 3-11-005962-2 , p. 420.
- ↑ W. Lossen: About the structural formula of hydroxylamine and its amide-like derivatives . In: Justus Liebig's Annals of Chemistry . tape 175 , no. 3 , 1875, p. 271-304 , doi : 10.1002 / jlac.18751750303 .