Light stick
A glow stick (also known as a glow stick ) is a purely chemical light source and is based on the principle of chemiluminescence . It consists of a transparent plastic container in which two liquids are located in separate chambers. The plastic container is filled with a solution of different chemicals, the composition of this liquid depending on the desired lighting duration and color.
history
Bis (2,4,5-trichlorophenyl-6-carbopentoxyphenyl) oxalate (CPPO) was developed by Michael M. Rauhut, Robert W. Sombathy and Laszlo J. Bollyky on the basis of the work of Edwin A. Chandross ( Bell Labs ) and Richard D Sokolowski (Eh.M Labs) and used for chemiluminescence. At the same time, Herbert Richter was conducting similar experiments at the China Lake Naval Weapons Center .
Richard Taylor Van Zandt is the registered inventor of US Patent 4064428, issued November 1, 1976 for "Chemical Light Device".
The US Department of Defense uses over 15 million glow sticks annually.
The largest light stick in the world, three meters high and 88.6 liters of liquid, was "bent" by Knixs GmbH on June 29, 2009 in Darmstadt .
construction
The light stick contains a fluorescent compound (fluorophore, fluorescer) and two other chemicals involved in the chemiluminescence reaction, one of which is enclosed in a glass tube. The plastic container itself contains an oxalic acid ester ( diphenyl oxalate (DPO, Cyalume) or its derivatives ) mixed with the fluorescer . The most commonly used ester component is:
When testing many different components, the best results were achieved with TCPO and DNPO, since trichlorophenol and dinitrophenols are good leaving groups that favor the nucleophilic attack of the hydrogen peroxide on the two carbonyl groups . A phthalic acid ester (e.g. dimethyl phthalate ) or ethyl acetate is usually used as the solvent for the ester .
The glass tube contains a 30% hydrogen peroxide solution. If the glass tube is broken, for example by kinking the rod, a chemical reaction sets in, the peroxyoxalate chemiluminescence .
Chemical reaction
When the glow sticks are activated, a complex sequence of chemical reactions is set in motion that have not yet been clarified in every detail; More details under the keyword peroxyoxalate chemiluminescence .
The glow ( luminescence ) is created when the fluorescer, excited by the chemical reaction, finally emits the energy in the form of the emission of a photon .
The wavelength of the photon, ie the color , depends on the structure of the fluorescer used. The reaction is not reversible, but it can be slowed down considerably by cooling. Luminous sticks usually shine in monochrome, with the colors red, yellow, orange, green, violet, blue, infrared and white (then of course not monochrome) available. The rod shape is not mandatory. They are also available as rings or in other shapes that can be made from a rod.
In the laboratory, this reaction can be greatly accelerated by adding sodium hydroxide , or better yet, the weakly basic sodium salicylate . The reaction then takes place in minutes or even seconds instead of hours, depending on the amount. For obvious reasons, however, this application is impractical for light sticks.
The glow time of glow sticks ranges from a few minutes to a few days (depending on size). Used glow sticks fluoresce under ultraviolet light ( " black light " ), since the fluorescer can also be excited by photons of higher energy than that of the emitted photons. This effect is not limited to UV, but occurs e.g. B. also on a red fluorescer, which is irradiated with green light.
Commonly used fluorescers
| Surname | Short name | Emission color | Structural formula | comment |
|---|---|---|---|---|
| Dihydro-9,10-diphenylanthracene | Dihydro (DPA) | violet | ||
| 9,10-diphenylanthracene with 2,4-di-tert-butylphenyl-1,4,5,8-tetracarboxynaphthalene diamide | pink | emits pink or white, depending on the mix ratio | ||
| 9,10-diphenylanthracene | DPA | blue |

|
|
| 1-chloro-9,10-diphenylanthracene | 1-chloro-DPA | blue |
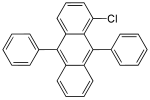
|
|
| 2-chloro-9,10-diphenylanthracene | 2-chloro-DPA | blue |

|
|
| 9,10-bis (phenylethynyl) anthracene | BPEA | green |

|
|
| 2-chloro-9,10-bis (phenylethynyl) anthracene | 2-chloro-BPEA | green |

|
|
| 1-chloro-9,10-bis (phenylethynyl) anthracene | 1-chloro-BPEA | yellow-green |

|
|
| 1,8-dichloro-9,10-bis (phenylethynyl) anthracene | 1,8-dichloro-BPEA | yellow |

|
|
| 5,6,11,12-tetraphenylnaphthacene | Rubren | yellow |
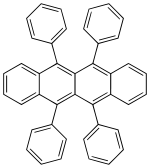
|
|
| 5,12-bis (phenylethynyl) naphthacene | orange |

|
||
| Rhodamine 6G | red |
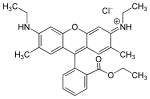
|
||
| (9- (2-carboxyphenyl) -3,6-bis (diethylamino) xanthylium chloride) | Rhodamine B | red |
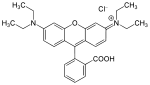
|
is rarely used because it breaks down together with phenyl oxalate |
| 2,4-di-tert-butylphenyl-1,4,5,8-tetracarboxynaphthalene diamide | deep red |
Areas of application
Light sticks serve as easily transportable emergency lighting , for marking or simply as an accessory . Skin contact or even ingestion of the now often non-toxic, but coloring and sometimes foul-smelling or tasting chemical substances should be avoided (see dangers ).
Often light sticks are also used in the area of fishing in order to be able to observe the swimmer or the tip of the fishing rod even in the dark . There are also golf balls that can be equipped with a small light stick so that you can play in the dark. Other areas of application are in the military and security sector, for example as emergency lighting, to mark the injured or those who have gone overboard , helicopter landing pads and to instruct aircraft.
Archers appreciate the carrot-sized variant for marking targets and the small fishing sticks for attaching to arrows for night shooting.
Furthermore, small glow sticks can be accommodated inside helium- filled (transparent) balloons , whereby balloons with one or two glow sticks inside can still fly without any problems and allow a very effective night start of a balloon mail .
There are also glow sticks on the market that emit radiation in the infrared range that cannot be perceived by the human eye. With the use of a night vision device , such a light stick can be seen in the dark.
In the 1990s, glow sticks became very popular due to their use at techno parties. This is where the dance or movement form called "glowsticking" was created, in which glow sticks are moved primarily with the hands in a playful and creative way to the music. A modified form is the "glow stringing". The glow sticks are (on strings English. "Strings") attached to and, like playing with poi , a spectacular part kind in circular paths wound around the body. This is then a form of spinning , a sub-discipline of juggling .
hazards
Light sticks contain hydrogen peroxide , and phenol is also formed as a by-product of the reaction. The liquid should not be swallowed or get on the skin. If the liquid gets on the skin, it may cause slight skin irritation. In extreme cases, nausea or vomiting is possible. If the liquid gets into the eyes, rinse with water immediately and then consult an ophthalmologist immediately, as both hydrogen peroxide and phenol can be corrosive. The glass parts of the small ampoule can injure the eyes. The liquid in the light sticks can dissolve some types of plastic (e.g. polystyrene ). In addition, some of the chemicals in glow sticks are flammable. In extreme heat (e.g. fire) there is also the risk of an explosion with a shrapnel effect , as the liquid in the glass tube comes under pressure from the heat, which ruptures the same.
Luminous sticks from more recent production are sold with the attribute "non-toxic" (non-toxic).
Individual evidence
- ↑ Elizabeth Wilson: What's that stuff? Light Sticks Archived from the original on May 19, 2012. Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (Reprint) In: Chemical & Engineering News . 77, No. 3, January 18, 1999, p. 65. Retrieved April 10, 2008.
- ^ Richard D. Sokolowski: Electro House: attaining electro perfection through fluid luminescence . March 8, 2008 ( A new method using enhanced green fluorescent protein (EGFP) to determine grazing rate on live bacterial cells by protists ).
- ↑ Patent US4064428 : Chemical Light Device.
- ^ Steve Givens: The great glow stick controversy (Forum Section) , Student Life. July 27, 2005.
- ↑ Largest Glowstick: Guinness World Records




