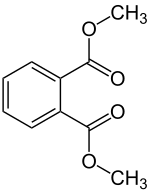
Dimethyl phthalate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dimethyl phthalate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 10 O 4 | ||||||||||||||||||
| Brief description |
light-sensitive, colorless, almost odorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 194.19 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.19 g cm −3 |
||||||||||||||||||
| Melting point |
6 ° C |
||||||||||||||||||
| boiling point |
282 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
slightly soluble in water (4.3 g l −1 ) |
||||||||||||||||||
| Refractive index |
1.5138 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 5 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Dimethyl phthalate (DMP) is a phthalic acid ester made from methanol and phthalic acid and belongs to the group of phthalates in addition to diethylhexyl phthalate , di-isononyl phthalate , diethyl phthalate and dibutyl phthalate .
Presentation and extraction
The industrial production of dimethyl phthalate takes place in a two-stage synthesis from phthalic anhydride and methanol . The first step is rapid alcoholysis to form the phthalic acid half-ester. The second esterification step is slower as an equilibrium reaction and requires the use of acidic catalysts and, in order to complete the conversion, the distillative separation of the water formed.
properties
Dimethyl phthalate is a light-sensitive, colorless, less volatile, highly viscous (dynamic viscosity of 1.7 mPa · s at 20 ° C.) And almost odorless liquid that is sparingly soluble in water. It decomposes in the heat, producing carbon monoxide and carbon dioxide as well as irritating vapors and gases.
Dimethyl phthalate is considered a difficultly flammable liquid. Flammable vapor-air mixtures can form above the flash point . The compound has a flash point of 146 ° C. The explosion range begins at 0.9 vol.% As the lower explosion limit (LEL). The lower explosion point is 134 ° C. The ignition temperature is 555 ° C. The substance therefore falls into temperature class T1.
use
DMP is used as a plasticizer , as a repellent against insects, where it is particularly effective against Anopheles , and as a solvent in glow sticks ("glow stick").
Individual evidence
- ↑ Entry on DIMETHYL PHTHALATE in the CosIng database of the EU Commission, accessed on February 11, 2020.
- ↑ a b c d e f g h i j k l m n o p Entry for CAS no. 131-11-3 in the GESTIS substance database of the IFA , accessed on June 28, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-208.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 131-11-3 or dimethyl phthalate ), accessed on November 2, 2015.
- ↑ a b P.M. Lorz, FK Towae, W. Enke, R. Jäckh, N. Bhargava, W. Hillesheim: Phthalic Acid and Derivatives in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007 .a20_181.pub2 .
- ↑ a b E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) p. 75, ISBN 978-3-8348-1245-2 .
- ↑ Military Preventive Medicine: Mobilization and Deployment, Vol 1.Chapter 22: Personal Protection Measures Against Arthropods, p. 508.
