Rubren
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
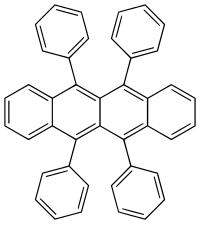
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Rubren | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 42 H 28 | |||||||||||||||
| Brief description |
red powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 532.67 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
315 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rubren ( 5,6,11,12-tetraphenylnaphthacene ) is a red polycyclic aromatic hydrocarbon which is used as a fluorescent dye in light sticks, among other things . As a result of chemiluminescence, it emits yellow light in this case.
Rubren is an organic semiconductor and is used as the starting material for the production of organic light-emitting diodes (OLED) and organic field effect transistors . At 40 cm 2 / (V · s), Rubren has the highest charge carrier mobility for defect electrons among organic semiconductors. The high mobility, however, is only achieved in perfectly formed Rubren crystals. In layers processed by thermal evaporation or from solution ( spin coating , printing processes), Rubren shows one to two orders of magnitude less mobility and is therefore largely due to other semiconducting materials, e.g. for work on organic field effect transistors . B. substituted acenes have been displaced.
Syntheses
Rubrene can be obtained from the adduct of phenylacetylene with benzophenone by treatment with thionyl chloride (cf. adjacent scheme). A preparative regulation for this route has found its way into the textbook literature. Regarding the mechanistic course of the reaction, it has long been assumed that a Diels-Alder reaction takes place between two of the intermediately formed chloro-triphenyl allenes (inclusion of a benzene ring with loss of aromaticity , structural elements in the adjacent scheme highlighted in bold). However, an improved understanding of the dimerization of allenes suggests that the dimerization proceeds radically via a bis-allyl diradical. Corresponding studies were carried out by Papagni and Yassar et al. released. The intermediate diradical then cyclizes with inclusion of a benzene ring, followed by HCl elimination with rearomatization. The naphthalene derivative formed has favorable stereoelectronic conditions for a 1,6-electrocyclic ring closure, again followed by HCl elimination with aromatization.
Access to rubrene derivatives with different aryl substituents on the tetracene structure are u. a. to that of Allen and Gilman, Dodge, Bain and Chamberlin, and Douglas et al. described routes are accessible (see adjacent diagram). In their approaches, Allen and Gilman use the conjugate addition of aryl Grignard reagents to tetracene-5,12-dione and the addition of aryl-Li compounds to the keto groups of tetracene-5,12-dione. The two variants of Dodge, Bain and Chamberlin are based on the construction of the tetracene structure by Diels-Alder reactions of isobenzofurans. Douglas et al. Finally, 6,11-dichloro-tetracene-5,12-dione is specifically functionalized by Suzuki coupling with boronic acids, followed by the addition of aryl-Li reagents to the keto groups of the tetracenedione.
Individual evidence
- ↑ a b c data sheet Rubrene, sublimed grade, 99.99% trace metals basis from Sigma-Aldrich , accessed on August 8, 2011 ( PDF ).
- ↑ Entry on 5,6,11,12-tetraphenylnaphthacene at ChemicalBook , accessed August 7, 2011.
- ↑ Tatsuo Hasegawa and Jun Takeya: Organic field-effect transistors using single crystals . (free download review) In: Sci. Technol. Adv. Mater. . 10, No. 2, 2009, p. 024314. bibcode : 2009STAdM..10b4314H . doi : 10.1088 / 1468-6996 / 10/2/024314 .
- ^ A b John E. Anthony: Functionalized Acenes and Heteroacenes for Organic Electronics. In: Chemical Reviews. 106, No. 12, 2006, pp. 5028-5048, doi: 10.1021 / cr050966z .
- ^ John E. Anthony: The Larger Acenes: Versatile Organic Semiconductors. In: Angewandte Chemie International Edition. 47, No. 3, 2008, pp. 452-483, doi: 10.1002 / anie.200604045 .
- ↑ Sybille Allard, Michael Forster, Benjamin Souharce, Heiko Thiem, Ullrich Scherf: Organic Semiconductors for Solution-Processable Field-Effect Transistors (OFETs). In: Angewandte Chemie International Edition. 47, No. 22, 2008, pp. 4070-4098, doi: 10.1002 / anie.200701920 .
- ^ First works: Charles Dufraisse, Bulletin de la Société chimique de France, 4e sér. 53, 1933, pp. 789-849.
- ^ Georg Wittig, Dieter Waldi: About a simplified representation of rubrics. In: Journal for Practical Chemistry. 160, No. 8-9, 1942, pp. 242-244, doi: 10.1002 / prac.19421600802 .
- ↑ a b B. S. Furniss, AJ Hannaford, PWG Smith, AR Tatchell (Eds.): Vogel's Textbook of Practical Organic Chemistry . 5th edition, Longman Scientific & Technical, 1989, ISBN 0-582-46236-3 , Chapter 6.1.4, Experiment 6.13 ( online access ).
- ^ A b C. FH Allen, L. Gilman: A Synthesis of Rubrene. In: Journal of the American Chemical Society. 58, No. 6, 1936, pp. 937-940, doi: 10.1021 / ja01297a028 . The Diels-Alder reaction is referred to here as "diene synthesis".
- ^ LF Fieser, M. Fieser: Organische Chemie . 2nd, improved edition, Verlag Chemie, Weinheim 1982, p. 1384.
- ↑ Joseph J. Gajewski, Chung Nan Shih: Hydrocarbon Thermal Degenerate Rearrangements. V. Stereochemistry of the 1,2-Dimethylenecyclobutane Self-Interconversion and Its Relation to the Allene Dimerization and the Rearrangements of Other C 6 H 8 Isomers. In: Journal of the American Chemical Society. 94, No. 5, 1972, pp. 1675-1682, doi: 10.1021 / ja00760a041 .
- ↑ Sarah L. Skraba, Richard P. Johnson: A Computational Model for the Dimerization of Allene. In: The Journal of Organic Chemistry. 77, No. 24, 2012, pp. 11096-11100, doi: 10.1021 / jo302176k .
- ↑ Daniele Braga, Abdelhafid Jaafari, Luciano Miozzo, Massimo Moret, Silvia Rizzato, Antonio Papagni, Abderrahim Yassar: The Rubrenic Synthesis: The Delicate Equilibrium between Tetracene and Cyclobutene. In: European Journal of Organic Chemistry. 2011, No. 22, 2011, pp. 4160-4169, doi: 10.1002 / ejoc.201100033 .
- ↑ Daniele Braga, Abdelhafid Jaafari, Luciano Miozzo, Massimo Moret, Silvia Rizzato, Antonio Papagni, Abderrahim Yassar: The Rubrenic Synthesis: The Delicate Equilibrium between Tetracene and Cyclobutene. In: European Journal of Organic Chemistry. 2011, No. 22, 2011, pp. 4160-4169, doi: 10.1002 / ejoc.201100033 .
- ↑ Kathryn A. McGarry, Wei Xie, Christopher Sutton, Chad Risko, Yanfei Wu, Victor G. Young, Jean-Luc Brédas, C. Daniel Frisbie, Christopher J. Douglas: Rubrene-Based Single-Crystal Organic Semiconductors: Synthesis, Electronic Structure, and Charge-Transport Properties. In: Chemistry of Materials. 25, No. 11, 2013, pp. 2254-2263, doi: 10.1021 / cm400736s .


