Luminol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
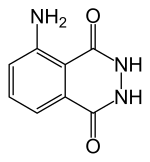
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Luminol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 7 N 3 O 2 | ||||||||||||||||||
| Brief description |
pale yellow crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 177.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
319-320 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Luminol is a yellowish to green shimmering, water-insoluble solid chemical compound , which is formally derived from aniline , phthalic acid and hydrazine .
Extraction and manufacture
Luminol is produced by nitrating phthalic acid to 3-nitrophthalic acid, converting it with hydrazine to 3-nitrophthalic acid hydrazide and then reducing it with sodium dithionite to give luminol.
Alternatively, the synthesis of 3-nitrophthalhydrazide from 3-nitrophthalic anhydride is possible, which gives better yields.
use
Criminology

Luminol is used in criminology to search for clues , even the smallest amounts of blood can be detected with it. The evidence is based on the fact that luminol reacts with oxidizing agents (mostly hydrogen peroxide ) to emit bluish light ( chemiluminescence ). This reaction only proceeds sufficiently quickly in the presence of a catalyst (here complexly bound Fe 2+ or Fe 3+ ). H. in practice, in the absence of catalysts, no reaction or no chemiluminescence can be observed.
To search for clues, two solutions are made: a solution of luminol in sodium hydroxide solution and a dilute hydrogen peroxide solution. These are put together shortly before use and the surfaces to be examined are sprayed. If there are traces of blood on it, the blood pigment hemoglobin (contains heme , an iron complex) catalyzes the reaction described below. So there is a bluish chemiluminescence. The test is sensitive enough to detect tiny amounts of blood in the urine, which makes urine stains appear "positive" too. However, the reaction is also catalyzed by copper ions, which thus interfere with this test.
The sensitivity can be varied by adding further substances: In a series of tests it was shown that active ingredients in the dishwashing detergent Fit intensify the luminescence, but also shorten it. The reason for this could be detergents in the cleaning agent, which make the plasma membranes of the erythrocytes more permeable, so that the luminol comes into contact with the Fe 2+ more quickly .
Luminol also enables tests for species identification and blood grouping. In addition, Luminol has no destructive effects on DNA , so that it can be analyzed using PCR .
Biochemical and medical analysis
Luminol chemiluminescence is also used in biochemical and medical analysis. Here z. B. so-called reactive oxygen species (ROS) in tissues, extracts and body fluids directly determined by the intensity of the luminol chemiluminescence. Another important analytical method is based on the fact that peroxidases are also able to catalyze the luminol reaction. Numerous immunoassays are based on this principle , with which extremely small amounts of toxins, pathogens, peptides and proteins can be detected. The most important peroxidase used for this is horseradish peroxidase (HRP, horseradish peroxidase). You can test the catalytic effect of horseradish peroxidase yourself by dripping a few drops of an alkaline luminol / hydrogen peroxide solution onto a piece of fresh horseradish.
Chemiluminescent reaction
In the chemiluminescence reaction, luminol is a starting material, a dicarbonyl compound, a so-called 1,4- dione .
The substance is first dissolved in sodium hydroxide solution, whereby luminol reacts as an acid with H + elimination with the hydroxide ions of the sodium hydroxide solution. This reaction is a reversible equilibrium reaction .
The resulting dianions are stabilized by mesomerism .
The actual reaction begins with the oxidation of the dianions by hydrogen peroxide or another oxidizing agent. In addition to molecular nitrogen, a phthalate , the 3-aminophthalate dianion , is formed as an unstable intermediate in the excited triplet state (T 1 ). The triplet state is due to two electrons with identical spin in an orbital of an oxygen atom (2S + 1 = 3 with S = 1 = 2 * 1/2, see multiplicity ). A radiationless transition causes the molecule to spin-flip into an isoenergetically excited singlet state (S 1 ).
The high-energy oxidized luminol molecule also serves as a sensitizer in this chemiluminescence. The transition of the electron from the more highly excited singlet state to the singlet ground state (S 0 ) causes a photon to be emitted in the blue range of the light spectrum .
See also
Web links
Individual evidence
- ^ Arnold Willmes, Pocket Book Chemical Substances , Harri Deutsch, Frankfurt (M.), 2007.
- ↑ Data sheet 3-aminophthalhydrazide from Acros, accessed on February 24, 2010.
- ^ A b c Mary Eagleson: Concise Encyclopedia Chemistry. Walter de Gruyter, 1994. ISBN 9783110114515 . P. 607.
- ↑ a b Luminol data sheet from Sigma-Aldrich , accessed on November 25, 2019 ( PDF ).
- ^ Entry on Luminol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ HDK Drew, FH Pearman: Chemiluminescent organic compounds. Part II. The effect of substitutes on the closure of phthalhydrazides to 5- and 6-membered rings , J. Chem. Soc., 1937, 26-33.
- ↑ Katrin Heuser, Martin Oehmen, Nadine Kühner and Mark Benecke: Effect of the “Fit” washing-up liquid on luminol fluorescence . In: Archives for Criminology . No. 217 , 2006, pp. 137-145 ( online ).
- ↑ Katrin Heuser, Martin Oehmen, Nadine Kühner and Mark Benecke: Effect of the "Fit" detergent on luminol fluorescence . In: Archives for Criminology . No. 217 , 2006, pp. 144 ( online ).
- ↑ Neha Passi, Rakesh Kumar Garg, Mukesh Yadav, Ram Sarup Singh, Magdy A. Kharoshah: Effect of luminol and bleaching agent on the serological and DNA analysis from bloodstain . In: Egyptian Journal of Forensic Sciences . tape 2 , no. 2 , 2012, p. 54-61 , doi : 10.1016 / j.ejfs.2012.04.003 .
- ^ Steffen Albrecht, Herbert Brandl, Thomas Zimmermann, chemiluminescence, Hüthig Verlag, Heidelberg 1996.

