Difluorodisulfane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
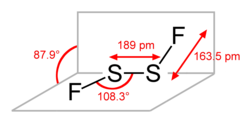
|
||||||||||
| General | ||||||||||
| Surname | Difluorodisulfane | |||||||||
| Molecular formula | F 2 S 2 | |||||||||
| Brief description |
colorless gas with an unpleasant odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 102.12 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| density |
4.3 kg m −3 |
|||||||||
| Melting point |
−133 ° C |
|||||||||
| boiling point |
−15 ° C |
|||||||||
| solubility |
reacts with water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Difluorodisulfane is a chemical compound between fluorine and sulfur and is isomeric to thiothionyl fluoride .
Extraction and presentation
Difluorodisulfan can be obtained by reacting silver (I) fluoride with sulfur at 125 ° C.
properties
Difluorodisulfan is a colorless gas with an unpleasant odor. It converts to the more stable thiothionyl fluoride at higher temperatures and pressures and then further to sulfur tetrafluoride and sulfur. Even at room temperature, the compound converts to thiothionyl fluoride in the presence of hydrogen fluoride or sodium fluoride. Unlike thiothionyl fluoride, difluorodisulphane reacts with nitrogen dioxide to form nitrosyl fluorosulphate .
Individual evidence
- ↑ a b c d Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 704 f . ( limited preview in Google Book search).
- ^ A b Hans Peter Latscha, Helmut Alfons Klein: Inorganic Chemistry . Springer DE, 2002, ISBN 3-540-42938-7 , pp. 341 ( limited preview in Google Book search).
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 379.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 182.

