Disulfur dinitride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Disulfur dinitride | |||||||||
| Molecular formula | S 2 N 2 | |||||||||
| Brief description |
colorless solid with an iodine- like odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 92.14 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
2.2 g cm −3 |
|||||||||
| Vapor pressure |
0.01 mbar (25 ° C) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Disulfur dinitride is an inorganic chemical compound of sulfur from the group of covalent nitrides . In addition to pentasulfur hexanitride , tetrasulfur dinitride , tetrasulfur tetranitride , monosulfur mononitride , oligosulfur dinitrides and polymeric polythiazyl (SN) x , the compound belongs to the group of sulfur-nitrogen compounds or sulfur nitrides .
Extraction and presentation
Disulfur dinitride can be obtained by reacting tetrasulphur tetranitride with silver (the resulting silver sulfide acts as a catalyst in the decomposition of tetrasulfur tetranitride to disulfur dinitride) or the thermal cleavage of tetrasulfur tetranitride.
properties
Disulfur dinitride is a colorless, crystalline, highly volatile solid with a disgusting iodine- like odor. It is only stable at low temperatures and turns dark after a short time at 20 ° C. The polymeric polythiazyl (SN) x is formed via a radical, paramagnetic intermediate . Explosive decomposition occurs in the event of impact or temperatures above 30 ° C. It sublimes at 10 −2 Torr even at room temperature. In the presence of traces of atmospheric moisture, about 67% polymerize to (SN) x , while 33% dimerize to tetrasulfur tetranitride. It is soluble in alcohols with a yellow-red color and easily dissolves in benzene , ether , carbon tetrachloride , acetone , tetrahydrofuran and 1,4-dioxane without coloring . Traces of alkali metals trigger instantaneous and exclusive dimerization. Water does not wet disulfur dinitride, which is why it is not soluble in water.
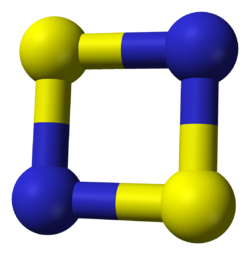
Disulfur dinitride is the smallest binary S — N ring system that has been isolated to date. The planar, almost square molecule with D 2h molecular structure has formally 6 π electrons and belongs to the pseudoaromatic compounds . However, it is not an aromatic, but has a singlet diradical character with four π electrons and two non-bonding π radical electrons. The bond lengths are 1.651 Å and 1.659 Å, the NSN and SNS bond angles are 89.9 ° and 90.4 °.
Solid disulfur dinitride has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . The compound forms with Lewis acids such as boron trichloride , aluminum trichloride and antimony pentachloride Lewis acid-base adducts of various compositions such. B. S 2 N 2 • BCl 3 , S 2 N 2 • 2AlCl 3 , S 2 N 2 • SbCl 5, and S 2 N 2 • 2SbCl 5 .
Individual evidence
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 275.
- ↑ a b Roger Blachnik (Ed.): Pocket book for chemists and physicists . Volume III: Elements, Inorganic Compounds and Materials, Minerals . founded by Jean d'Ans, Ellen Lax. 4th, revised and revised edition. Springer, Berlin 1998, ISBN 3-540-60035-3 , pp. 706 ( limited preview in Google Book search).
- ↑ a b c d e f Wiberg, E .; Wiberg, N .; Holleman, AF: Inorganische Chemie , 103rd edition, 2017 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-026932-1 , p. 681, (accessed via De Gruyter Online).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1905.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Alsfasser, R .; Janiak, C .; Klapötke, TM ; Meyer, H.-J .: Modern Inorganic Chemistry , editor Riedel, E., 3rd edition 2007, Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-019060-1 , p. 129 –132, (accessed via De Gruyter Online).
- ↑ Ralf Alsfasser, HJ Meyer: Moderne Anorganische Chemie . Walter de Gruyter, 2007, ISBN 3-11-019060-5 , p. 123 ( limited preview in Google Book search).
- ↑ Entry on sulfur-nitrogen compounds. In: Römpp Online . Georg Thieme Verlag, accessed on March 2, 2017.
