Pentasulfur hexanitride
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
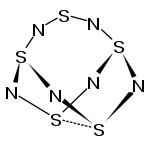
|
|||||||
| General | |||||||
| Surname | Pentasulfur hexanitride | ||||||
| Molecular formula | S 5 N 6 | ||||||
| Brief description |
orange crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 244.34 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Pentasulfur hexanitride is an inorganic chemical compound of sulfur from the group of covalent nitrides . The compound is one next to the disulfur dinitride , the Tetraschwefeldinitrid , the tetrasulfur tetranitride , the Monoschwefeldinitrid , the Monoschwefelmononitrid , the Oligoschwefeldinitriden and the polymeric polythiazyl (SN) x to the group of sulfur-nitrogen compounds or Schwefelnitride .
Production is achieved by reacting tetrasulfur tetra nitrogen dichloride with bis (trimethylsilyl) sulfur diimide.
Another synthetic route is the reaction of bromine with tetrabutylammonium tetrasulfur pentanitride Bu 4 N + S 4 N 5 - in methylene chloride .
The compound forms orange crystals that are prone to explosive decay. It is stable at room temperature under inert conditions . When air enters, it turns black immediately. Thermal decomposition begins above 130 ° C. In vacuum at 10 -2 mbar the compound at 45 ° C can be sublimated be.
The structure of the pentasulfur hexanitride can be derived from that of the tetrasulfur tetranitride S 4 N 4 by incorporating a sulfur diimide group into an SS bond. The molecular structure can be seen as basket-like with the S 4 N 4 part of the molecule as the actual basket and the NSN group as the associated handle. The SN bond lengths are on average 161 pm in the S 4 N 4 moiety, 152.6 pm in the NSN moiety and 170.6 pm at the points of attachment. By incorporating a further sulfur diimide group into the second SS bond, a hexasulfur octanitride S 6 N 8 could be obtained, which has not yet been described in the literature.
Individual evidence
- ↑ a b c d e f g h i Wiberg, E .; Wiberg, N .; Holleman, AF: Inorganische Chemie , 103rd edition, 2017 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-026932-1 , p. 682, (accessed via De Gruyter Online).
- ↑ a b c Entry on sulfur-nitrogen compounds. In: Römpp Online . Georg Thieme Verlag, accessed on March 2, 2017.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
