Tetrasulfur tetranitride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Tetrasulfur tetranitride | |||||||||
| Molecular formula | S 4 N 4 | |||||||||
| Brief description |
orange crystals (25 ° C ) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 184.287 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
2.22 g cm −3 |
|||||||||
| Melting point |
178.2 ° C |
|||||||||
| boiling point |
185 ° C |
|||||||||
| solubility |
almost insoluble in water, soluble in carbon disulfide |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Tetrasulfur tetranitride is an inorganic chemical compound of sulfur from the group of covalent nitrides . The compound is one next to the Pentaschwefelhexanitrid , the Tetraschwefeldinitrid , the disulfur dinitride , the Monoschwefelmononitrid , the Oligoschwefeldinitriden and the polymeric polythiazyl (SN) x to the group of sulfur-nitrogen compounds or Schwefelnitride .
history
The compound was first produced in contaminated form by M. Gregory in 1835 by reacting disulfur dichloride with ammonia . The stoichiometry could not be specified until 1850. The tetrameric nature was recognized by R. Schenck in 1896 . The molecular structure as an eight-membered ring was proposed in 1936 and confirmed by M. Goehring from 1947 onwards . In 1952 and 1963, the angled structure of the eight-membered ring as a cage connection was characterized by means of X-ray diffraction measurements . Tetrasulfur tetranitride is thus one of the longest known cage compounds.
Extraction and presentation
Tetrasulfur tetranitride can be made by reacting sulfur with liquid ammonia in the presence of silver nitrate . The added silver ions serve to intercept the hydrogen sulfide formed as silver sulfide and shift the equilibrium to the product side.
A number of synthesis variants start from disulfur dichloride. For example, the compound can be obtained by reaction with ammonia in dichloromethane and subsequent recrystallization in toluene .
The synthesis in chlorine- saturated carbon tetrachloride or dichloromethane at 20–50 ° C or with ammonium chloride without solvent at 150–160 ° C works better . Various sulfur nitride chlorides such as the thiazyl chloride ClSN and S 3 N 2 Cl 2 , S 4 N 3 Cl and S 3 N 3 Cl 3 occur in the synthesis sequence .
Another synthesis is based on sulfur tetrachloride , which is produced from sulfur dichloride and sulfuryl chloride and reacted with a substituted diaminosulfane.
properties
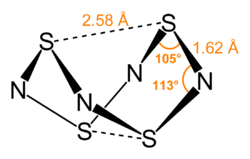
Tetrasulfur tetranitride is a cage compound whose structure corresponds to a D 2d symmetry. The structure can be seen as a tetrahedron of electropositive sulfur atoms penetrated by a square of electronegative nitrogen atoms. All SN bonds are of the same length at 162 pm, so that multiple bonds with delocalized π electrons can be concluded. In addition, the closed cage structure contains two weak SS bonds with a bond length of 258 pm. This bond length is much longer than that of the single bonds in the S 8 molecule with 205 pm, but much shorter than that of a Van der Waals distance with 360 pm. The compound is thermochromic , which means that it changes color from colorless (at 77 K), through bright orange (at 298 K) to red (at 373 K). Tetrasulfur tetranitride sublimes below 130 ° C at 0.1 mbar pressure. It is insoluble in water. Solid tetrasulphur tetranitride decomposes explosively into its elements when heated to over 130 ° C or when impacted, with a heat of decomposition of −460 kJ / mol.
Gaseous tetrasulphur tetranitride decomposes above 200 ° C. mainly into the ring-shaped dimer disulfur dinitride , with S 3 N 3 and S 4 N 2 also being formed. Above 300 ° C it breaks down into the elements and sulfur mononitride SN. The hydrolysis in a strongly basic medium gives ammonia , thiosulphate and sulphite .
In a weakly basic medium, trithionate , ammonia and sulfite are formed. Organic bases do not completely cleave the S 4 N 4 ring. The reaction with Grignard compounds RMgBr gives the product (R = aryl) or an addition compound with trimethylsilyldimethylamine .
With strong acids such as tetrafluoroboric acid , protonation occurs on a nitrogen atom with salt formation.
The compound forms addition compounds with Lewis acids such as. B. Antimony pentachloride as S 4 N 4 · SbCl 5 , with titanium tetrachloride as S 4 N 4 · TiCl 4 , with tin tetrachloride as (S 4 N 4 ) 2 · SnCl 4 , with boron trifluoride as S 4 N 4 · BCl 3 , with arsenic pentafluoride as S 4 N 4 · AsF 5 , with sulfur trioxide as S 4 N 4 · SO 3 , with tantalum pentachloride as S 4 N 4 · TaCl 5 , with copper (I) chloride as S 4 N 4 · (CuCl) 2 or with Iron (III) chloride as S 4 N 4 · FeCl 3 . The adduct with aluminum chloride S 4 N 4 · (AlCl 3 ) 4 represents an adduct S 2 N 2 · (AlCl 3 ) 2 , since the tetrasulfur tetranitride was split up by the aluminum chloride to form disulfur dinitride.
use
Tetrasulphur tetranitride is one of the most important starting materials for the production of sulfur-nitrogen compounds. The reaction with silver (II) fluoride results in a fluorination of the sulfur atoms, whereby the eight-membered ring is retained.
With mercury (II) fluoride trimer is thiazyl fluoride (NSF) 3 or with chlorine the Thiazylchlorid formed.
A reduction takes place on the nitrogen atom. The eight-membered ring is retained in the tetrasulfur tetraimide formed . The compound resembles the S 8 ring, with every second S atom being replaced by an NH group. The reduction with hydrogen iodide is complete and leads to ammonia and hydrogen sulfide.
A controlled thermal decomposition results in the disulfur dinitride , which is further converted into the polythiazyl .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p E. Wiberg , N. Wiberg , AF Holleman : Inorganische Chemie . 103rd edition. Walter de Gruyter, Berlin / Boston 2017, ISBN 978-3-11-026932-1 , pp. 676–680, (accessed via De Gruyter Online).
- ↑ a b c d Entry on sulfur-nitrogen compounds. In: Römpp Online . Georg Thieme Verlag, accessed on March 2, 2017.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ M. Gregory: in Z. Pharm. 21, 1835, p. 315 and 22, 1835, p. 301.
- ↑ J.-M. Fordos, A. Gélis: Memoire on the sulfure d'azote. In: Compt. Rend. 31, 1850, pp. 702ff.
- ^ R Schenck: in Liebigs Ann. Chem. 290, 1896, p. 171.
- ^ MHM Arnold, JAC Hugill, JM Hutson: The formation and constitution of sulfur nitride and so-called hexasulphamide. In: J. Chem. Soc. (London) 1936, pp. 1645-1649, doi: 10.1039 / JR9360001645 .
- ^ MHM Arnold: The structure of (SNH) 4 and its derivatives. In: J. Chem. Soc. (London) 1938, pp. 1596-1597, doi: 10.1039 / JR9380001596 .
- ↑ M. Goehring: About the sulfur nitrogen N 4 S 4 . In: Chem. Ber. 80, 1947, pp. 110-122, doi: 10.1002 / cber.19470800204 .
- ↑ M. Goehring, J. Ebert: A chemical evidence for mesomerism in tetrasulfur tetranitride. In: Z. Naturforsch. B 10, 1955, pp. 241–244, (pdf)
- ↑ D. Clark: The structure of sulfur nitride. In: J. Chem. Soc. 1952, pp. 1615-1620, doi: 10.1039 / JR9520001615 .
- ↑ BD Sharma, J. Donohue: The Crystal and Molecular Structure of Sulfur Nitride, S 4 N 4 . In: Acta Cryst. 16, 1963, pp. 891-897, doi: 10.1107 / S0365110X63002401 .
- ↑ a b c C. Janiak, H.-J. Meyer, D. Gudat, R. Alsfasser: Riedel - Moderne Anorganische Chemie. 4th edition. Walter de Gruyter, Berlin / Boston 2012, ISBN 978-3-11-024900-2 , pp. 129–130, (accessed via De Gruyter Online).
- ↑ a b c d e f Ralf Steudel : Chemistry of Non-Metals, Syntheses - Structures - Binding - Use. 4th edition. Walter de Gruyter, Berlin / Boston 2014, ISBN 978-3-11-030439-8 , pp. 515-519, (accessed from De Gruyter Online).
- ↑ Hans-Georg Elias: Macromolecules . tape 3 : Industrial Polymers and Syntheses . John Wiley & Sons, 2009, ISBN 978-3-527-62652-6 , pp. 515 ( limited preview in Google Book search).
- ↑ a b c Lothar Kolditz : Inorganic Chemistry. Deutscher Verlag der Wissenschaften, Berlin 1983, p. 480f.




![{\ displaystyle \ mathrm {2 \ SCl_ {4} +2 \ [(Me_ {3} Si) _ {2} N] _ {2} S \ longrightarrow S_ {4} N_ {4} +8 \ Me_ {3 } SiCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4999c4f622a4218626915496351102ead71ad771)











