Tetrasulfur dinitride
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Tetrasulfur dinitride | ||||||
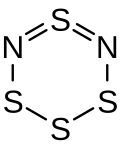
| Molecular formula | S 4 N 2 | ||||||
| Brief description |
dark red solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 156.28 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
1.71 g cm −3 (supercooled melt) |
||||||
| Melting point |
23 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tetrasulfur dinitride is an inorganic chemical compound from the group of covalent nitrides . In addition to pentasulfur hexanitride , tetrasulfur tetranitride , disulfur dinitride , monosulfur mononitride , oligosulfur dinitrides and polymeric polythiazyl (SN) x , the compound belongs to the group of sulfur-nitrogen compounds or sulfur nitrides .
history
The compound was first established in 1897 by Wilhelm Muthmann and E. Clever, the compound being mistaken for a nitrogen pentasulfide S 5 N 2 . In the early literature, various chain and ring structures were postulated for the compound. The sum formula S 4 N 2 could be determined by A. Meuwsen in 1951 by means of cryoscopy . The real structure as a six-membered ring with the nitrogen atoms in the 1- and 3-positions was discovered in 1971 by Henry G. Heal using 15 N- NMR spectroscopy , IR spectroscopy , Raman spectroscopy , UV-VIS spectroscopy , mass spectrometry and dipole measurements proven.
Extraction and presentation
Tetrasulfur dinitride can be obtained by reacting tetrasulphur tetranitride with sulfur at 110 ° C.
A second synthetic route that avoids the explosive tetrasulphur tetranitride starts with heptasulphurimide, which is converted into the mercury salt by means of mercury (II) acetate . This then breaks down into mercury (II) sulfide , tetrasulfur dinitride and sulfur.
properties
Tetrasulfur dinitride is a less stable diamagnetic dark red solid which decomposes within a few hours at 0 ° C. At 100 ° C explosive decomposition to sulfur and nitrogen takes place . The compound is soluble in many organic solvents such as. B. benzene , nitrobenzene , carbon disulfide , carbon tetrachloride and diethyl ether , only moderately soluble in alcohols, but insoluble in water. A slow hydrolysis takes place with water, which is considerably accelerated in the basic medium. The compound is relatively stable to dilute acids.
The tetrasulfur dinitride molecule forms a non-planar six-membered ring in a half-chair shape , with the atoms S-N = S = N-S lying in one plane and bridged by a sulfur atom lying above this plane.
A stable adduct with the composition S 4 N 2 · 2BF 3 is formed with boron trifluoride . This only begins to decompose above 240 ° C.
Individual evidence
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 604.
- ↑ a b c d e f g h Goehring, M .; Herb, H .; Wissemaier, H .: About the tetrasulfur dinitride S 4 N 2 in Z. anorg. allg. Chem. 267 (1952) 238-246, doi : 10.1002 / zaac.19522670406 .
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 404.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Muthmann, W .; Clever, E .: About the nitrogen pentasulfide in Z. anorg. gen. Chem. 13 (1897) 200-208, doi : 10.1002 / zaac.18970130123 .
- ↑ Meuwsen, A .: About the tetrasulfur dinitride S 4 N 2 in Z. anorg. allg. Chem. 266 (1951) 250-255, doi : 10.1002 / zaac.19512660409 .
- ↑ Nelson, J .; Heal, HG: Structure of tetrasulphur dinitride in J. Chem. Soc. A, 1971, 136-139, doi : 10.1039 / J19710000136 .
- ↑ Heal, HG; Ramsay, RJ: A new, safe preparation of tetrasulphur dinitride in J. Inorg. Nucl. Chem. 37 (1975) 286, doi : 10.1016 / 0022-1902 (75) 80172-2 .
- ↑ Zhu, J.-K .; Gimarc, BM: Structure of tetrasulfur dinitride in Inorg. Chem. 22 (1983) 1996-1999, doi : 10.1021 / ic00156a014 .


