Mercury (II) acetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
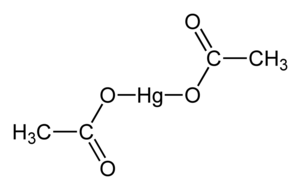
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Mercury (II) acetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 HgO 4 | |||||||||||||||
| Brief description |
white solid with a slight vinegar odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 318.7 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.27 g cm −3 |
|||||||||||||||
| Melting point |
178–180 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
0.1 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mercury (II) acetate is a chemical compound , more precisely the mercury salt of acetic acid with the constitutional formula Hg (CH 3 COO) 2 .
Extraction and presentation
Mercury (II) acetate can be produced by reacting acetic acid with mercury (II) oxide .
use
Mercury (II) acetate is used as a catalyst and for the production of organic mercury compounds (e.g. phenyl mercury (II) acetate , 2-methoxyethyl mercury chloride ). A special form is the oxymercuration - demercuration .
Web links
Commons : Mercury (II) Acetate - Collection of pictures, videos, and audio files
Individual evidence
- ↑ a b c d e f g h Entry on mercury (II) acetate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Dimethyl Sulfoxide (DMSO) Solubility Data. Gaylord Chemical Company, LLC; Bulletin 102, June 2014, p. 14. (PDF)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling falls under the group entry inorganic compounds of mercury with the exception of mercuric sulphide and those specified elsewhere in this Annex in the Classification and Labeling Inventory of European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Mention of mercury acetate in determining the fat number (PDF; 346 kB)
- ↑ Whitmore FC, Hanson ER: o-Chloromercuriphenol In: Organic Syntheses . 4, 1925, p. 13, doi : 10.15227 / orgsyn.004.0013 ; Coll. Vol. 1, 1941, p. 161 ( PDF ).
- ↑ Entry on (2-methoxyethyl) mercury chloride. In: Römpp Online . Georg Thieme Verlag, accessed on October 10, 2018.
- ↑ Entry on Oxymercuration -Demercuration on ChemPage.de



