Diallyl disulfide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diallyl disulfide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 S 2 | |||||||||||||||
| Brief description |
yellowish clear liquid with an intense garlic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.28 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.0 g cm −3 |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
1.3 h Pa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diallyl disulfide (DADS), a chemical compound from the group of organic disulfides , is a foul-smelling yellowish liquid that is insoluble in water. It arises from the decomposition of allicin , which is released when garlic and other plants of the leek family (Alliaceae) are cut. Many of the health-promoting effects of garlic can be ascribed to diallyl disulfide, but also the allergy-causing effects. Diluted it is used as a flavoring in the food industry.
history
In 1844 Theodor Wertheim found the presence of the allyl group he had named and of sulfur in garlic oil , which he obtained directly from garlic by steam distillation . But it was not until 1892 that Friedrich Wilhelm Semmler was able to identify diallyl disulfide as one of the components of the oil. The precursor allicin was discovered in 1944 by Chester J. Cavallito and John Hays Bailey as a malodorous, easily decomposable yellow liquid. In 1947 Arthur Stoll and Ewald Seebeck found that DADS and allicin are produced in situ from cysteine derivatives by the enzyme alliinase .
Occurrence
Diallyl disulfide is produced by decomposition of allicin , which with the injury of cells of the leek plants (especially garlic , onion and leek ) from alliin , using the enzyme alliinase is released. The highest content of DADS could be found in the steam distillation of garlic bulbs; here DADS makes up about a quarter of the sulfur-containing fraction, which takes up about two percent by weight in the plant. But the leaves of the garlic are also a source of the substance; their oil, which makes up about 0.06 percent of the undried plant material, contains about one third diallyl disulfide.
Extraction and presentation
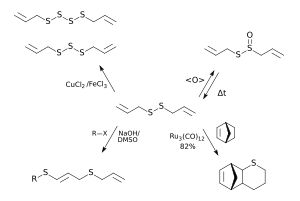
Diallyl disulfide can be prepared on an industrial scale from sodium disulfide and allyl chloride or bromide at temperatures between 40 and 60 ° C. under an inert gas , the sodium disulfide being generated in situ from sodium sulfide and sulfur . The reaction is exothermic and a yield of 88 percent of what is theoretically possible was achieved.
In smaller quantities, the synthesis in air can also be carried out catalytically with the aid of TBAB from the same starting materials; the yield here is up to 82 percent.
Both in the synthesis and in the extraction from plant material, the main problem for the preparation in pure form is the contamination with diallyl polysulfides. Since the mixture is difficult to separate, only 80% diallyl disulfide is usually available commercially. This can be purified by distillation under reduced pressure; if you work carefully, you get a clear (not yellowish) liquid with a characteristic garlic odor.
The reduction of allicin to diallyl disulfide takes place particularly quickly in the pure state above 37 ° C. In aqueous solutions with a pH between 1.2 and 7.5, only 10 percent of the allicin was converted after five hours at this temperature. Only traces of allicin are detectable in the blood after just five minutes.
properties
Physical Properties
DADS smells intensely of garlic with a metallic note. The clear yellowish liquid boils at 138-139 ° C (applies to the 80 percent raw substance), has its flash point at 50 ° C, a density of around 1.0 g / ml and has a vapor pressure of 1 mm Hg at 20 ° C on. The good fat solubility and the insolubility in water can be explained by the lack of polarity of the molecule.
Chemical reaction
See figure on the right:
- Diallyl disulfide can be oxidized to allicin , from which it is formed by dissociation .
- Catalytic reaction to diallyl polysulphides.
- Addition to alkyl halides leads to 1-alkylthio-3-allylthio-1-propenes and 1,3-di (alkylthio) propenes.
- DADS is a possible participant in ruthenium complex -catalyzed cyclization reactions that lead to sulfur-containing hetero-polycycles.
use
DADS can be used as a starting point for the synthesis of higher diallyl polysulfides ; it is catalyzed with iron chloride / copper chloride . It also serves as the starting product for the new synthesis of allicin ; since this is unstable, it is produced shortly before use (for example for ajoene synthesis ). The food industry uses DADS to improve the taste of meat, vegetables and fruits.
Biological importance
The area of activity of diallyl disulfide in biological systems covers several areas, whereby a special ion channel is activated in the lowest concentration , which in humans provides the characteristic smell and taste of the substance. Interventions in the detoxification system are the main reactions within the cells . There is also evidence of specific mechanisms of action within cancer cells.
When consumed, hydrogen sulfide is partially produced in the stomach . At the same time, diallyl disulfide is metabolized in the liver to other biologically active substances, including allyl methyl sulfoxide (AMSO), allyl methyl sulfone (AMSO (2)) and allyl methyl sulfide (AMS), and it is reoxidized to allicin. The breakdown product AMS is what is responsible for the later content of acetone in the breath, and thus part of the bad breath . Sometimes (up to 3 hours after consumption) it is also DADS itself that is noticeable in the breath.
Smell and taste
The cause of the unpleasant smell and the sharp taste that many living beings perceive in the presence of DADS is TRPA1 . This ion channel is significant in developmental biology and can already be found in fungi. The leek plants obviously selected for the effect of diallyl disulfide on TRPA1 at an early stage in order to protect themselves from invasive enemies such as fungi and later from predators such as animals.
Conversely, the garlic taste and smell of food preparations can be neutralized by adding special foods such as kiwi , spinach , parsley , basil , edible mushrooms and especially cow's milk , raw chicken eggs , cooked rice and beef serum albumin . The reaction concerns the diallyl disulfide and is enzymatic in the case of spinach and asparagus .
Detoxification and poisoning
Many of the effects of diallyl disulfide derive from the interventions in the cell detoxification system, in particular the antimicrobial, mutation-inhibiting, cancer-preventing and anti-cancer effects, and protection against cardiovascular diseases .
The main target of diallyl disulfide is the part of the cell's detoxification mechanism called 'Phase II' .
Particularly noteworthy is the role in detoxification using the GSH / GST system: DADS significantly increases the production of the enzyme glutathione-S-transferase (GST) in cells. This normally mediates the binding of glutathione (GSH) to electrophilic toxins in the cell. Garlic therefore supports the detoxification function of liver cells in vitro and protects nerve cells from oxidative stress in vitro. At the same time, DADS reacts directly and dose-dependently with GSH (to allyl glutathione sulfide), which in cells that are exposed to larger amounts of DADS means that, conversely, more reactive oxygen species are formed, which ultimately lead to cell death (so-called oxidative stress ). High doses of DADS can, for example, destroy the protective effect in mouse nerve cells in vitro and have the opposite effect.
The fact that the detoxification effect also has a preventive effect and can prevent inflammatory symptoms in the intestine became apparent in a study in which rats were treated with endotoxin after prolonged intake of DADS and were then protected from damage to intestinal cells by the poison. This study also made it clear that certain side effects of garlic oil in high doses cannot be ascribed to diallyl disulfide.
By supporting the detoxification activity in the liver, diallyl disulfide can theoretically be used as liver protection during chemotherapy . As a polysulphide, it is a substrate for cyanide ions and is therefore theoretically directly and indirectly suitable for cyanide detoxification. There are no studies on humans.
Antimicrobial effect
The organic sulfur compounds released when plant cells are destroyed are of great benefit to the leek family thanks to their antimicrobial, insect-repellent and larvicidal properties . Humans have made these properties their own for thousands of years.
DADS is the effective principle of garlic oil in inhibiting the growth of mold , gram-positive and gram- negative bacterial strains . However, the allicin and the higher-quality polysulfides in garlic seem to be even more effective against the gastric ulcer germ Helicobacter pylori . In a diabetes model in mice, several infection markers ( CRP , TNF , IL-6 ) could be normalized after systemic infection with MRSA by repeated administration of diallyl disulfide . In the rat model of Pneumocystis jirovecii infections, DADS can replace cotrimoxazole with the same efficiency. In the case of the Candida yeast , DADS leads to oxidative stress and cell death by producing a glutathione deficiency , blocking the electron transport chain and mitochondrial ATP synthase . In a retrospective observational study, the antifungal effects were the presumed cause of a significantly reduced risk of ventilated intensive care patients to develop fungal infections of the lower respiratory tract.
Because of its antimicrobial effects, diallyl disulfide and tobramycin are part of a preparation for preoperative selective intestinal decontamination. In a clinical study, their benefits in preventing endotoxemia in heart valve operations were proven.
Protection against colon cancer
In the laboratory model, garlic can prevent the development of colon cancer. Various studies have supported the assumption that diallyl disulfide is one of the ingredients of garlic that can play a role in preventing and inhibiting cancer by interfering with multiple levels of cell metabolism. These effects are mostly dose-dependent and this dose-dependency explains why in vivo consumption of garlic primarily affects colon cancer cells. In contrast, by injecting the substance, leukemia cells can also be reached, as was shown in the mouse model.
The mode of action of DADS on cancer cells differs from that on normal cells: Cancer cells are much more sensitive to the substance and initiate cell death more quickly. DADS also leads, depending on the dose, to a sudden, strong accumulation of reactive oxygen species and thus, and by activating further enzymes, to the start of the apoptosis program of cancer cells. However, as was demonstrated on a cell line , this effect can be prevented by an increased amount of glutathione and the production of glutathione peroxidase in the cells. By acetylating histones in cancer cells, DADS can also prevent tumor growth and spread in a dose-dependent manner. This could be shown in both colon cancer and leukemia cells in rats in vivo. Finally it could be shown that the substance intervenes directly in the cell cycle of human colon cancer cells. Cancer is caused, among other things, by the accumulation of reactive oxygen species in the cell when the normal detoxification mechanisms of the cell are no longer sufficient. Diallyl disulfide influences the part of the cell metabolism that takes care of the detoxification of the cell and helps, in small amounts, with detoxification and thus with the prevention of cancer (see detoxification and poisoning ).
Protection against cardiovascular diseases
There is some evidence that garlic can prevent the development of cardiovascular diseases. One possible cause of some of these diseases, such as arteriosclerosis or coronary artery disease , is oxidative stress , which diallyl disulfide reduces by helping to detoxify the cell. DADS is therefore partly responsible for the mentioned effects of garlic. However, this is not the substance's only mechanism of action.
In a clinical study, diallyl disulfide reduced the surrogate markers atherosclerotic plaques and CRP. However, the literature on the subject of diallyl disulfide and blood lipid levels in general is contradictory.
By activating the TRP ion channel TRPA1, DADS leads to a short-term decrease in blood pressure.
Other effects
DADS activates the transport protein ferritin in the iron metabolism , as was shown in vivo in rats.
In another animal experiment, rats fed a diet high in protein increased their testosterone levels when they were fed garlic powder . At the same time, the plasma content of corticosterone decreased . A significant increase in luteinizing hormone caused by diallyl disulfide is likely the cause.
safety instructions
DADS irritates the skin and triggers allergies , and in particular it is the cause of garlic and onion allergies in cooks , where it mostly occurs on the fingertips. Protective gloves cannot prevent this.
For diallyl disulfide there is an oral LD 50 value of 260 mg / kg in rats or the value of 3,600 mg / kg for dermal absorption. Applied to the skin of cats in high doses of 5 g / kg, it was fatal to haemolytic anemia . In connection with this, there have been reports of pet and rat poisoning from large amounts of garlic.
proof
Even traces of DADS in the air or in the blood can easily be detected by gas chromatography .
Web links
- Organosulfur Compounds From Garlic ( Memento from February 12, 2008 in the Internet Archive )
- Spiegel Online: Garlic is said to defeat Campylobacter
Individual evidence
- ↑ a b c d e f data sheet Allyldisulfide at The Good Scents Company .
- ↑ a b c d entry to diallyl disulfide in the GESTIS Bank of IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c data sheet Allyl disulfide from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).
- ↑ Nishimura, H .; Mizutani, J .: Photochemistry and radiation chemistry of sulfur-containing amino acids. New reaction of the 1-propenylthiyl radicals in J. Org. Chem. 40 (1975), 1567-1575, doi: 10.1021 / jo00899a011 .
- ↑ Higuchi, O .; Tateshita, K .; Nishimura, H .: in J. Agric. Food Chem. 51: 7208-7214 (2003).
- ↑ Chester J. Cavallito, John Hays Bailey: Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. In: Journal of the American Chemical Society. 66, 1944, p. 1950, doi : 10.1021 / ja01239a048 .
- ^ A b Arthur Stoll, Ewald Seebeck: Chemical Investigations on Alliin, the Specific Principle of Garlic. In: Advanced Enzymology. 11, 1951, pp. 377-400. doi : 10.1002 / 9780470122563.ch8 .
- ↑ LD Lawson et al .: Identification and HPLC Quantitation of the Sulfides and Dialk (en) yl Thiosulfinates in Commercial Garlic Products. In: Planta Med 57 / - 1991 , pp. 363-370. doi: 10.1055 / s-2006-960119
- ↑ AE Edrois and HM Fadel: Investigation of the volatile aroma components of garlic leaves essential oil. Possibility of utilization to enrich garlic bulb oil. European Food Research and Technology. 214/2 2002 , pp. 105-107. doi: 10.1007 / s00217-001-0429-2
- ↑ Patent WO2006016881 : Process for Producing Diallyl Disulfide. Published on February 16, 2006 , inventor John R. Maloney, Kevin J. Theriot, Sharon Booth D. McGee, James E. Torres, Woodrow R. Wilson Jr
- ^ Yuan Xin-ke et al .: Synthesis, characterization and bioactivity evaluation of diallyl disulfide. J. of Central South University of Technology. 13/5 2006 , pp. 515-518. doi: 10.1007 / s11771-006-0079-4
- ↑ F. Freeman and Y. Kodera: Garlic Chemistry: Stability of S-2-Propenyl-2-Propene-1-sulfinothioate (Allicin) in Blood, Solvents, and Simulated Physiological Fluids. J Agric Food Chem. 43 1995 , pp. 2332-2338, doi: 10.1021 / jf00057a004 .
- ↑ Amosova SV et al .: Synthesis of 1-alkylthio-3-allylthio-1-propenes by the reaction of diallyl disulfide with allyl halides in the alkali-metal hydroxide-DMSO superbasic system. J. Org. Chem. USSR (Engl. Transl.) 22/5 1986 , pp. 957-963, doi: 10.1002 / chin.198636161 ( abstract ).
- ↑ Kondo T. et al .: Ruthenium Complex-Catalyzed Novel Addition-Cyclization Reaction of Allylic Disulfides with 2-Norbornenes. Nippon Kagakkai Koen Yokoshu. 76/2 1999 , p. 922 ff. Abstract
- ↑ Patent US5231114 : Polysulfides compounds and lipid peroxidation inhibitor containing the polysulfide compounds as active ingredient. Published July 27, 1993 , Inventors: Shoji Awazu, Toshiharu Horie, Yukihiro Kodera, Shinji Nagae, Hiromichi Matsuura, Yoichi Itakura.
- ↑ E. Germain et al .: In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotics . 32/12 2002 , pp. 1127-1138. PMID 12593760
- ↑ a b E. Germain et al .: Hepatic metabolism of diallyl disulphide in rat and man. Xenobiotics. 33/12 2003 , pp. 1185-1199. PMID 14742141
- ↑ DL Lawson and ZJ Wang: Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J Agric Food Chem. 53/6 2005 , pp 1974-1983. PMID 15769123 .
- ↑ Atta ur Raman: Studies in Natural Products Chemistry. Bioactive Natural Products, Part D. Elsevier Science, 2000 . ISBN 0-444-50606-3 , p. 466 ff.
- ^ A b D. M. Bautista et al .: Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA . 102/34 2005 , pp. 12248-12252, doi: 10.1073 / pnas.0505356102 (free full text).
- ↑ AG Hile et al .: Aversion of European starlings (Sturnus vulgaris) to garlic oil treated granules: Garlic oil as an avian repellent. J Agric Food Chem. 52/8 2004 , pp. 2192-2196. PMID 15080619
- ^ O. Negishi et al .: Effects of food materials on removal of Allium-specific volatile sulfur compounds. J Agric Food Chem. 50/13 2002 , pp. 3856-3861. PMID 12059171
- ↑ CW Tsai et al .: Garlic organosulfur compounds upregulate the expression of the pi class of glutathione S-transferase in rat primary hepatocytes. J Nutr. 135/11 2005 , pp. 2560-2565. PMID 16251611
- ↑ CC Wu et al .: Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem. 50/2 2002 , pp. 378-383. PMID 11782211
- ↑ T. Fukao et al .: The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol . 42/5 2004 , pp. 743-749. PMID 15046820 .
- ↑ a b K. M. Lemar et al .: Diallyl disulphide depletes glutathione in Candida albicans: oxidative stress-mediated cell death studied by two-photon microscopy. Yeast. 24/8 2007 , pp. 695-706. PMID 17534841 .
- ↑ Y. Hu et al .: Glutathione- and thioredoxin-related enzymes are modulated by sulfur-containing chemopreventive agents. Biol Chem. 388/10 2007 , pp. 1069-1081. PMID 17937621 .
- ↑ SH Koh et al .: Protective effect of diallyl disulfide on oxidative stress-injured neuronally differentiated PC12 cells. Brain Res Mol Brain Res. 133/2 2005 , pp. 176-186. PMID 15710234
- ↑ Kim JG et al .: Differential effects of diallyl disulfide on neuronal cells depend on its concentration. In: Toxicology . 211 / 1-2 2005 , pp. 86-96. PMID 15863251
- ↑ YH Chiang et al .: Effects of garlic oil and two of its major organosulfur compounds, diallyl disulfide and diallyl trisulfide, on intestinal damage in rats injected with endotoxin. In: Toxicol Appl Pharmacol . 213/1 2006 , pp. 46-54. PMID 16274720
- ↑ M. Iciek et al .: Selective effects of diallyl disulfide, a sulfane sulfur precursor, in the liver and Ehrlich ascites tumor cells. In: Eur. J. Pharmacol. 569 / 1-2 2007 , pp. 1-7. PMID 17560567 .
- ↑ M. Iciek et al .: Allyl disulfide as donor and cyanide as acceptor of sulfane sulfur in the mouse tissues. In: Pharmacol Rep . 57/2 2005 , pp. 212-218. PMID 15886420
- ↑ S. V Amonkar and A. Banerji: Isolation and characterization of larvicidal principle of garlic. Science . 174/16 1971 , pp. 1343-1344. PMID 5135721
- ↑ Avato P et al .: Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicins. 7/3 2000 , pp. 239-243. PMID 11185736
- ^ EA O'Gara et al .: Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 66/5 2000 , pp. 2269-2273. PMID 10788416
- ↑ SM Tsao et al .: Two diallyl sulphides derived from garlic inhibit meticillin-resistant Staphylococcus aureus infection in diabetic mice. J Med Microbiol. 56/6 2007 , pp. 803-808. PMID 17510266
- ^ Lu ZM et al .: [The experimental study of garlicin in treating Pneumocystis carinii pneumonia in rats]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. (Chinese journal of parasitology & parasitic diseases). 22/6 2004 , pp. 357-360. PMID 15830863
- ↑ J. Guan et al .: [Preventive effect and clinical significance of garlicin injection on mechanical ventilator-associated low respiratory tract deep-seated fungal infection]. Zhongguo Zhong Xi Yi Jie He Za Zhi (Chinese Journal of Alternative Medicine). 24/6 2004 , pp. 505-507. PMID 15250201
- ↑ J. Yu et al .: Effects of preoperatively selected gut decontamination on cardiopulmonary bypass-induced endotoxemia. Chin. J. Traumatol. 10/3 2007 , pp. 131-137. PMID 17535634
- ^ World Cancer Research Fund / American Institute for Cancer Research: Food, Nutrition, Physical Activity, and the Prevention of Cancer . 2nd edition, 2007 ( ISBN 0-9722522-2-3 ) pp. 93-94
- ↑ John A. Milner: Preclinical Perspectives on Garlic and Cancer. J. Nutr. 136 / - 2006 , pp. 827S-831S. Online version
- ↑ JS Yang et al .: Diallyl disulfide inhibits WEHI-3 leukemia cells in vivo. Anticancer Res . 26 / 1A 2006 , pp. 219-225. PMID 16475702
- ↑ Z. Huang et al .: Bcl-2 small interfering RNA sensitizes cisplatin-resistant human lung adenocarcinoma A549 / DDP cell to cisplatin and diallyl disulfide. Acta Biochim Biophys Sin (Shanghai). 39/11 2007 , pp. 835-843. PMID 17989874
- ↑ A. Das et al .: Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 110/5 2007 , pp. 1083-1095. PMID 17647244 .
- ↑ G. Filomeni et al .: Glutathione-related systems and modulation of extracellular signal-regulated kinases are involved in the resistance of AGS adenocarcinoma gastric cells to diallyl disulfide-induced apoptosis. Cancer Res. 65/24 2005 , pp. 11735-11742, PMID 16357186 .
- ↑ MC Myzak and RH Dashwood: Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets . 7/4 2006 , pp. 443-452. PMID 16611031
- ↑ N. Druesne-Pecollo et al .: In vivo treatment by diallyl disulfide increases histone acetylation in rat colonocytes. 354/1 2007 , pp. 140-147. PMID 17210128
- ↑ Jie Zhao et al .: Diallyl disulfide suppresses growth of HL-60 cell through increasing histone acetylation and p21 WAF1 expression in vivo and in vitro. In: Acta Pharmacologica Sinica 27/11 2006 , pp. 1459-1466. doi: 10.1111 / j.1745-7254.2006.00433.x
- ↑ HJ Jo et al .: Diallyl disulfide induces reversible G2 / M phase arrest on a p53-independent mechanism in human colon cancer HCT-116 cells. Oncol. Rep. 19/1 2008 , pp. 275-280. PMID 18097607
- ↑ WL Cheng et al .: Clinical study on the effect of Garlicin in stabilizing the carotid artery atherosclerotic plaque in patients with primary hypertension and coronary artery disease. Chin J Integr Med. 12/3 2006 , pp. 166-170. PMID 17005074 .
- ↑ M. Thomas et al .: Diallyl Disulfide Increases Rat H-Ferritin, L-Ferritin and Transferrin Receptor Genes In Vitro in Hepatic Cells and In Vivo in Liver. J. Nutr. 132 / - 2002 , pp. 3638-3641. Online version
- ↑ Y. Oi et al .: Garlic supplementation increases testicular testosterone and decreases plasma corticosterone in rats fed a high protein diet. J Nutr. 131/8 2001 , pp. 2150-2156. PMID 11481410
- ↑ M. Moyle et al .: Use of gloves in protection from diallyl disulphide allergy. In: Australasian Journal of Dermatology . 45/4 2004 , pp. 223-225. PMID 15527433 .
- ↑ EPA documents
- ^ Documents of the US Department of Labor Occupational Safety & Health
- ↑ X. Sun et al .: Simultaneous determination of diallyl trisulfide and diallyl disulfide in rat blood by gas chromatography with electron-capture detection. Pharmazie 61/12 2006 , p. 985. PMID 17283653 .