Lots
The substance group of mustards includes a number of chlorinated organic sulfur - or nitrogen-containing compounds, and some of these substances as is mainly due to the use of chemical weapons known. The often simply as Lost or mustard gas called sulfur Lost is the best known representatives of this group.
The name Lost goes back to the first letters of the surnames of the two German chemists who proposed the use of sulfur mustard as a chemical warfare agent in 1916, Wilhelm Lo mmel and Wilhelm St einkopf , both of Fritz Haber's employees at the Kaiser Wilhelm Institute for physical and electrochemistry in Berlin-Dahlem.
properties
In their pure form, mustards are liquid at room temperature. N-mustard, here HN-3, was mostly used as a solid hydrochloride .
In the First World War, mustards were used as warfare agents. The release took place as a liquid or solid aerosol , when fired in grenades by their dismantling charge , otherwise in liquid form by a spray device (spray attack). Both S-Musts and N-Musts were designated as yellow cross warfare agents due to the labeling of the ammunition filled with them .
Lost penetrates porous material and some types of rubber and plastic. Enrichment with waxes, resins or plastics creates viscous mustards that stick to materials and are therefore more difficult to detoxify (so-called sedentary warfare agents).
Sulfur mustards (S-Mustard)
The sulfur mustards are terminally chlorinated thioethers . This group includes nine chemicals:
| chemical | code | Common name | CAS no. | PubChem | structure |
|---|---|---|---|---|---|
| Bis (2-chloroethyl) sulfide | H / HD | Lost, mustard gas | 505-60-2 | 10461 |
|
| 1,2-bis (2-chloroethylthio) ethane | Q | Sesqui-yperite | 3563-36-8 | 19092 |
|
| Bis (2-chloroethylthioethyl) ether | T | Oxol mustard | 63918-89-8 | 45452 |
|
| 2-chloroethylchloromethyl sulfide | 2625-76-5 |
|
|||
| Bis (2-chloroethylthio) methane | HK | 63869-13-6 |
|
||
| Bis-1,3- (2-chloroethylthio) - n -propane | 63905-10-2 |
|
|||
| Bis-1,4- (2-chloroethylthio) - n -butane | 142868-93-7 |
|
|||
| Bis-1,5- (2-chloroethylthio) - n -pentane | 142868-94-8 |
|
|||
| Bis (2-chloroethylthiomethyl) ether | 63918-90-1 |
|
Pure S-mustard is odorless and colorless; the typical garlic-like odor arises because S mustard is generally only synthesized in technical quality for practical reasons and the by-products and decomposition products present in traces resemble the odor of garlic ( allicin ). Furthermore, the technical product is mostly contaminated with colloidally dissolved sulfur and therefore appears milky to yellowish.
During the First World War , it was found that raw must obtained using the Oxol process was more toxic than pure must obtained differently. The reason for this was small amounts of Oxol mustard, which is significantly more toxic. This was only proven after the end of the war.
In addition to the normal S-Lost, the so-called Winterlost was also made during the First World War. To lower the freezing point, one gave the S-Lost z. B. added arsine oil or mixed it with other lots.
As further mixtures are u. a. known:
- HL (HD + Lewisite (L))
- HT (HD + T)
- HM (HT + Q + phenylarsine dichloride (PD))
- HQ (H + Q + arsine oil )
- HS (HD + HN)
Nitrogen mustard
The group of nitrogen mustards includes three tertiary, at least two terminally chlorinated amines :
| chemical | code | Common name | CAS no. | PubChem | structure |
|---|---|---|---|---|---|
| Bis (2-chloroethyl) ethylamine | HN-1 | Ethyl-S | 538-07-8 | 10848 |

|
| Bis (2-chloroethyl) methylamine | HN-2 | Mechlorethamine , chloromethine | 51-75-2 | 4033 |

|
| Tris (2-chloroethyl) amine | HN-3 | Trichloromethine | 555-77-1 | 5561 |
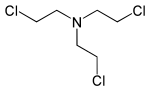
|
HN-1 was developed as a wart remover in the early 1930s; only later was its military usefulness revealed.
HN-2 was initially developed as a warfare agent. Later, it was used to produce a drug for chemotherapy against lymph node cancer ( chloromethine ).
toxicity
The main routes of exposure to mustards are percutaneous or inhalation absorption of vapors (only with mustard ). Lost is a powerful skin toxin and has been shown to be carcinogenic . The effect on the skin is comparable to severe burns or chemical burns . Large, very painful blisters form. The injuries heal badly. The tissue is permanently destroyed and cell division is inhibited. Extensively affected limbs usually have to be amputated. If the vapors are inhaled, the bronchi are destroyed.
The skin can be detoxified by immediate treatment, e.g. B. Wash the affected areas with soapy water or chlorinated lime slurry. Covering the affected areas of the body, for example with clothing or blankets, without prior detoxification makes the symptoms even worse.
The toxic effect of the mustard variants comes about through the formation of highly reactive compounds due to an intramolecular S N 2 attack of the nitrogen or sulfur on the carbon atom bound to the chlorine ( neighboring group effect), which in the case of nitrogen mustards results in an aziridinium ion , forms a thiiranium ion in the case of S- mustard .
These combine with amino groups (R-NH 2 ) of deoxyribonucleic acid ; a second - and in the case of N-Losten also a third - repetition of the deposition can lead to biochemically useless or toxic molecules. The modified DNA can, for example, trigger programmed cell death or cause cancer .
The eye is most sensitive to S-Lost vapor. The result is temporary blindness in the minor case, as the massive eyelid edema prevents active eye opening. The eyes are able to regenerate to a certain extent. Therefore, there are often good chances of recovery and the prospect of regaining sight, but this can take a few months.
Medical use of lost derivatives
Experience with the cell division inhibiting effect of sulfur mustard led to the fact that after the First World War the first cytostatics based on nitrogen mustard were developed and used in cancer therapy . However, the original warfare agents were still far too toxic for medical use. Examples of successful mustard-based cancer drugs are bendamustine , cyclophosphamide , ifosfamide , melphalan and chlorambucil .
N-mustard derivatives can alkylate other biological targets in addition to DNA (as in the case of the cytostatic group of “ alkylating agents ”). When attached to an active substance in a suitable position, the bis (2-chloroethyl) amino fragment can therefore be used as a linker via which ligands can bind covalently and irreversibly to biological targets such as receptors.
Examples of this are the irreversible binding opiate antagonist chlornaltrexamine (derived from naltrexamine) and the opiate agonist chloroxymorphamine (derived from oxymorphamine). The covalent bond results in an extremely long duration of action that can last up to 6 days.
Use as a chemical weapon
According to reports from Amnesty International , mustard ( mustard gas , lewisite or nitrogen mustard ) was allegedly used in several incidents near Darfur in Sudan and, according to speculation, several times against civilians in the 2010s during the civil war in Syria .
International controls
The mustards are listed as chemicals on list 1 in the international disarmament treaty CWC by the responsible UN authority, the Organization for the Prohibition of Chemical Weapons (OPCW) based in The Hague . Manufacture or possession is prohibited; This does not apply to work that only serves to protect against these substances. In Germany, any non-state handling of lots must be approved by the Federal Office of Economics and Export Control (BAFA) and reported to the OPCW.
Web links
Description of the controls in the international disarmament treaty CWC
Individual evidence
- ^ Florian Schmaltz: Warfare agent research under National Socialism: on the cooperation of Kaiser Wilhelm Institutes, the military and industry . Wallstein Verlag, 2005, ISBN 978-3-89244-880-8 , pp. 21 ( limited preview in Google Book search).
- ↑ bis (2-chloroethyl) sulfide in the lexicon of biology / chemistry . science-online
- ↑ Mechlorethamine Hydrochloride (English)
- ^ Effects of mustard gas . In: Medical Manual of Chemical Warfare (English).
- ↑ Blister Agent: Sulfur Mustard (H, HD, HS) ( Memento from March 11, 2009 in the Internet Archive ) (English).
- ↑ PS Portoghese et al .: 6beta- [N, N-bis (2-chloroethyl) amino] -17- (cyclopropylmethyl) -4,5alpha-epoxy-3,14-dihydroxymorphinan (Chloranaltrexamine), a Potent Opioid Receptor Alkylating Agent with Ultralong Narcotic Antagonist Activity . In: J. Med. Chem. Volume 21 , no. 598 , 1978.
- Jump up ↑ TP Caruso et al .: Chloroxymorphamine, and opioid receptor site-directed alkylating agent having narcotic agonist activity . In: Science . tape 204 , no. 316 , 1979.
- ↑ Sudan: Credible evidence of the use of chemical weapons to kill and maim hundreds of civilians including children in Darfur revealed . Amnesty International, September 29, 2016.
- ^ Alleged poison gas attack in Syria . tagesschau.de, April 5, 2017.
