Thiomargarita namibiensis
| Thiomargarita namibiensis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
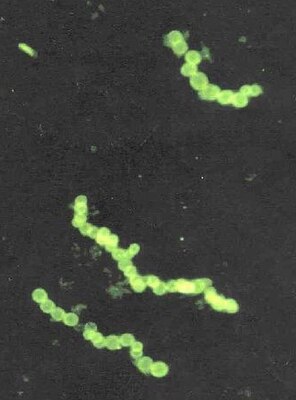
Thiomargarita namibiensis (Image: NASA) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Thiomargarita namibiensis | ||||||||||||
| Schulz et al., 1999 |
Thiomargarita namibiensis is a species of the gram-negative sulfur bacteria and the only representative of its genus fromthe Thiotrichaceae family . With diameters of up to 0.75 millimeters, these bacteria arethe largest known prokaryotic organisms in the world by volume . They are visible to humans with the naked eye. They occur exclusively on the sea floor directly on the coast of Namibia and, as so-called chemolithotrophic creatures,gaintheir energy from the conversion of inorganic substances, in particular sulfides with nitrate , which they store in their cell interior in high concentrations. In contrast to most sulfur bacteria, they can also convert the sulfides with oxygen under certain conditions, so they are facultatively aerobic . The species was first discovered by German researchers in 1997 and first described in 1999.
description
The cells are spherical or barrel-shaped, have a volume of up to 0.2 cubic millimeters (= 200 million cubic micrometers) and normally between 100 and 300 micrometers in diameter, but they also regularly reach sizes of up to 750 micrometers. An average of 4 to 20 cells connect through a mucous membrane to form straight or branched chains. With high population densities, several chains can in turn form spherical structures. Occasionally, in very dense populations, the formation of large, cauliflower-shaped aggregations of significantly smaller and more opaque cells could be observed. It is still unclear whether this is a survival stage of Thiomargarita or an independent, previously undescribed species.
Ultrastructural
Most of the individual cells, around 98%, are taken up by the liquid vacuole , which serves as a nitrate store . With regard to the ultrastructure , two things stand out:
The outer cytoplasmic layer is as in related species such as Beggiatoa spp. , only 0.5 to 2 micrometers thick and of an unusually loose, sponge-like structure; the sulfur globules embedded in them give the actually colorless cells a bright white color. In addition to the sulfur deposits , there are also significantly smaller globules, which are assumed to be polyphosphates or glycogen .
The distribution of nucleic acids within the cytoplasm is irregular and complex, the DNA forms small, regularly occurring clusters instead of being evenly distributed. A similar phenomenon has also been observed in Epulopiscium fishelsoni , another giant bacterium that was considered the largest known bacterium until the discovery of Thiomargarita . It is therefore believed possible that such groupings of nucleoids are necessary for controlling particularly large cells.
Multiplication
The cell division takes place generally along a strand. Often all cells of one cord are at the same stage of cell division, which makes synchronization of neighboring cells very likely. The layer of mucus between cells that have recently divided is still very thin, but it becomes thicker with age, which favors the breakdown of longer chains. Longer chains of 40 to 50 cells become brittle and break up into several parts, creating separate chains.
Distribution area
Thiomargarita namibiensis can only be found off the Namibian coast along an approximately 740 kilometer long and up to 76 kilometer wide coastal strip from the Skeleton Coast around Palgrave Point to Lüderitz Bay , with a focus on Cape Cross and Conception Bay . The Benguela Current , a cold ocean current that extends along the African Atlantic coast from the Cape of Good Hope to the Equator, forms an upwelling area here , creating an ecosystem that is particularly rich in species and rich in phytoplankton . In this zone, the sea floor is formed on an area of around 18,000 square kilometers from very liquid, green diatomaceous mud , a sediment made up of deposited diatoms . Here Thiomargarita occurs at depths of 100 meters in the upper 10 to 14 centimeters of the sediment and can reach population densities with a weight of up to 200 g / m². The highest density is reached directly below the sediment surface, to which extraordinarily large amounts of organic material (= detritus ) constantly sink. As a result of the decomposition of the organic material, almost no oxygen (0-3 micromoles / liter) and extremely high nitrate concentrations (over 10 micromoles / liter) occur on the sea floor for long periods .
Way of life
Only while thiomargarita are on the sediment surface or in the water column above can they absorb nitrate or possibly oxygen from the water. The chains get there through storms and gas eruptions that occur regularly in this region, but also through other, smaller events such as strong waves, which stir up the diatom sludge together with the chains stored in them (the so-called "resuspension").
The cell aggregates of Thiomargarita , which themselves are immobile, end up in the water column, where they absorb and store nitrate. They then sink back into deeper areas of the sediment and are sometimes covered for months. Nitrate or oxygen can no longer be found there, so they fall back on the highly concentrated nitrate stored in their vacuole. If the vacuole is completely filled, the nitrate reserve in the water is sufficient for around 40 to 50 days at normal metabolic rates. Beyond this time, Thiomargarita is also able to survive for several years without nutrients if necessary; isolated living cells are reported from culture after a storage period of over 4 years at 5 ° C and without the addition of nitrates or sulfides.
The sulphides, which are produced during the decomposition of the detritus by anaerobic , sulphate-reducing bacteria in the upper 20 centimeters of the sediment, are “breathed in” with the nitrate as electron acceptor ( nitrate respiration ). The sulfide is oxidized to elemental sulfur, which is stored in the cytoplasm in the form of small granules and reaches concentrations of 0.4 to 1.7 mol / liter total volume. It is not known whether the stored elemental sulfur thiomargarita, like other bacteria, can be used as an energy source and, under certain conditions, is further oxidized to sulfate .
metabolism
Thiomargarita has a very flexible metabolism: it can take place under reducing conditions ( anoxic ) as well as under oxidizing conditions ( oxic ).
The majority of the time, the immobile cells are under oxygen-free (anoxic) conditions in the sediment containing hydrogen sulfide. Under these conditions the sulfide acts as an electron donor and nitrate as an electron acceptor . The nitrate is reduced to ammonium or elemental nitrogen . Exactly the question of the end product cannot yet be answered, however, as the pure culture required for this has not yet been achieved. Assuming that the nitrate is reduced to ammonium, the sum equation looks like this :
Since there is no nitrate in this environment, the cells fall back on the nitrate stored in the vacuole.
When the sediment is mixed with seawater, the cells come into contact with oxygen and nitrate. In contrast to other sulfur bacteria, Thiomargarita tolerates oxygen up to the saturation limit. The uptake of sulfide is accelerated in the presence of oxygen. Under these conditions, oxygen acts as an electron acceptor. Much of the energy gain could come about during these relatively short phases. During these phases, the nitrate storage is also filled with energy consumption.
In addition to hydrogen sulfide and nitrate / oxygen, Thiomargarita also converts phosphate and acetate . Under oxic conditions they take up phosphate and store it in the high-energy form of polyphosphate (see also adenosine triphosphate ). Under anoxic conditions, the polyphosphate is broken down again with energy gain. Among other things, acetate is consumed for this purpose, which is presumably stored in the form of glycogen . The glycogen can then be inhaled again under oxic conditions.
ecology
The binding of sulfur by Thiomargarita and the Beggiatoa - and - rare - Thioploca -sulfur bacteria , which also occur there, has an important function in the ecosystem, because it prevents the release of hydrogen sulfide into the water, which is highly toxic to most living things. This is how they make the habitats habitable for oxygen-bound life forms. The consequences of breaking this bond were shown by natural methane gas eruptions on an extraordinarily large scale in the spring of 2001, which released large amounts of hydrogen sulfide from the sediment. Through this, all of the free oxygen in the water was bound on an area of over 20,000 square kilometers and at the same time oxygen-dependent aquatic organisms were poisoned, so that life in the water was largely killed or driven away. Such eruptions of the methane bubbles, some of which are only a few meters to decimeters below the surface, occur on a much smaller scale at least once a month and ensure the regular resuspensions of the sediment, which determine the rhythm of life of Thiomargarita .
Not least because of their size, thiomargarita are also a habitat for other types of bacteria. Occasionally, thread-like bacteria of the genus Thiotrix anchor themselves to the shell of the cells , but the mucous membrane of the chains in particular is the habitat of numerous species, including most likely species of the genus Desulfonema , which also colonize the mucous membranes of Thioploca .
Their active phosphate metabolism results in high phosphate concentrations (up to 300 micromoles per liter) in the pore water of the area inhabited by Thiomargarita . As a result, phosphorite forms in this zone (up to 5% of the dry matter of the sediment phosphorus), a rock that is used industrially as a starting material for the production of phosphorus and the exact origin of which was unclear for a long time. Today it is assumed that such deposits go back to former occurrences of bacteria with a similar metabolism.
Systematics

Thiomargarita namibiensis was found in 1997 by a research group at the Max Planck Institute for Microbiology Bremen under the direction of Heide Schulz when they examined sediment samples from the Walvis Bay on the coast of Namibia on board the Russian research vessel Petr Kottsov . The find was not a coincidence, but the expedition was looking specifically for representatives of this group due to the fact that the conditions here were very similar to those in other upwelling areas with occurrence of sulfur bacteria, but rather expected Thioploca and Beggiatoa species.
In 1999 the find was first described by Heide Schulz, the generic name Thiomargarita means something like 'sulfur pearl' and alludes to the strands of white, glossy, spherical cells that are reminiscent of pearl necklaces, the specific epithet refers to the Namibian coast as a place of discovery. A holotypical pure culture does not exist for technical reasons, but Thiomargarita can be kept in an enrichment culture without any problems . Heide Schulz contributed to the present with further research to the knowledge of the species and its living conditions.
Thiomargarita namibiensis is currently the only described representative of the genus; in 2005, however, a previously not formally described sulfur bacterium was discovered off the Mexican coast, which, according to molecular genetic studies, is 99% genetically identical to Thiomargarita namibiensis and is understood as a member of the genus in later work by other authors . At 180 to 375 micrometers, it reaches similar sizes, but does not live in chains, but appears as a single cell or cluster . This Thiomargarita namibiensis relative is also capable of what is known as “reductive cell division”: If the sulphide concentration in its habitat falls below a certain threshold value, the cell begins to divide into smaller units without increasing their size.
According to molecular genetic studies, Thiomargarita is closely related to the genus Thioploca , which fills similar ecological niches off the coasts of Chile and Peru, as well as the Beggiatoa . It is Thiomargarita as Thioploca ingrica within the beggiatoa positioned to see other studies also Thioploca araucae and Thioploca chileae as part of a sister group of Thiomargarita . The following cladogram follows Kalanetra et al., 2005 (simplified representation, names in bold represent taxon groups, the name is followed by the respective accession numbers of the sequence databases ):
| NN |
|
||||||||||||||||||||||||||||||
|
|
proof
- Heide N. Schulz: The Genus Thiomargarita , in: M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, E. Stackenbrandt (Ed.): The Prokaryotes , Volume 6., pp. 1156–1163, 2006, ISBN 978-0-387-33496-7 .
- Heide N. Schulz, Dirk de Beer: Uptake Rates of Oxygen and Sulfide Measured with Individual Thiomargarita namibiensis Cells by Using Microelectrodes. In: Applied And Environmental Microbiology. Volume 68, No. 11, 2002, pp. 5746-5749.
- Heide N. Schulz, Bo Barker Jørgensen: Big Bacteria. In: Annual Review of Microbiology. Vol. 55, 2001, pp. 105-137.
- Bernice Wuethrich: Giant Sulfur-Eating Microbe Found. In: Science, New Series. volume 284, No. 5413, 1999, p. 415.
- Heide N. Schulz, D. Riechmann: Thiomargarita namibiensis: A giant with staying power . In: BioSpektrum 6, 2000, pp 116-118.
Individual evidence
- ↑ a b H. N. Schulz; T. Brinkhoff; TG Ferdelman; M. Hernández Mariné; A. Teske; BB Jørgensen: Dense Populations of a Giant Sulfur Bacterium in Namibian Shelf Sediments , Science Vol. 284, No. 5413, 1999, pp. 493–495, Online ( Memento of the original dated February 5, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ^ Heide N. Schulz, Bo Barker Jørgensen: Big Bacteria , p. 117.
- ^ Heide N. Schulz: The Genus Thiomargarita , pp. 1158, 1159.
- ^ Heide N. Schulz, Bo Barker Jørgensen: Big Bacteria , p. 130.
- ^ Heide N. Schulz, Bo Barker Jørgensen: Big Bacteria , p. 131.
- ^ Heide N. Schulz, D. Riechmann: Thiomargarita namibiensis: A giant with long breath , p. 118.
- ↑ a b Volker Brüchert, Bronwen Currie, Kathleen R. Peard, Ulrich Lass, Rudolf Endler, Arne Dübecke, Elsabé Julies, Thomas Leipe, Sybille Zitzmann: Biogeochemical and physical control on shelf anoxia and water column hydrogen sulphide in the Benguel, a coastal upwelling system off Namibia. In: Past and Present Water Column Anoxia , NATO Science Series, 2006, Vol. 64, Part II, ISBN 978-1-4020-4262-1 , pp. 161-193.
- ↑ Heide N. Schulz, Dirk de Beer: Uptake Rates of Oxygen and Sulfide Measured with Individual Thiomargarita namibiensis Cells by Using Microelectrodes , in: Applied And Environmental Microbiology, Vol. 68, No. 11, 2002, pp. 5746-5749.
- ↑ “ ... even though most of the time T. namibiensis cells survive in sediments containing high sulfide concentrations with internally stored nitrate as their sole electron acceptor, they may be physiologically most active during times when the sediment is suspended. "Heide N. Schulz, Dirk de Beer: Uptake Rates of Oxygen and Sulfide Measured with Individual Thiomargarita namibiensis Cells by Using Microelectrodes. P. 5748.
- ^ Heide N. Schulz, Horst D. Schulz: Large Sulfur Bacteria and the Formation of Phosphorite. In: Science, Vol. 307, No. 5708, pp. 416-418.
- ↑ Scarla J. Weeks, Bronwen Currie, Andrew Bakun: Massive emissions of toxic gas in the Atlantic. In: Nature, 415, pp. 493-494, 2002
- ^ Heide N. Schulz: The Genus Thiomargarita , pp. 1160–1161.
- ^ Heide N. Schulz, Horst D. Schulz: Large Sulfur Bacteria and the Formation of Phosphorite. Pp. 416-418.
- ^ A whale of a bug. In: BBC News, April 15, 1999, online
- ↑ Validation List Number 71: Validation of publication of new names and new combinations previously effectively published outside the IJSB. In: International Journal of Systematic Bacteriology. 1999, 49, pp. 1325-1326.
- ^ A b c Karen M. Kalanetra, Samantha B. Joye, Nicole R. Sunseri, Douglas C. Nelson: Novel vacuolate sulfur bacteria from the Gulf of Mexico reproduce by reductive division in three dimensions. In: Environmental Microbiology 7 (9), 2005, pp. 1451-1460, PMID 16104867 .
- ↑ Andreas Teske: Fig. 9, in: Heide N. Schulz: The Genus Thiomargarita , p. 1160.
- ↑ For more information on this organism, which is not formally described, see: Karen M. Kalanetra, Sherry L. Huston, Douglas C. Nelson: Novel, Attached, Sulfur-Oxidizing Bacteria at Shallow Hydrothermal Vents Possess Vacuoles Not Involved in Respiratory Nitrate Accumulation. In: Applied and Environmental Microbiology. Volume 70, No. 12, 2004, pp. 7487-7496.
Web links
- Heide N. Schulz: Thiomargarita namibiensis: Giant Microbe Holding Its Breath. In: ASM News, 68: 3, 2002, pp. 122-127, Online PDF
- Daniela Riechmann: Upwelling areas of the oceans - all good things come from below. Dossier in: Scinexx - The Knowledge Magazine. July 14, 2003, online
- A Bloom By Any Other Name ... Might Never Be a Bloom At All , in: Science Focus! - SeaWiFS, March 29, 2007, online
- Entry on Thiomargarita namibiensis in the MicrobeWiki of Kenyon College (English)
- Image of a Thiomargarita namibiensis strand
- Image of Thiomargarita in size comparison with a fly



