Ferredoxins
| 2Fe-2S-ferredoxin ( Escherichia coli K12) | ||
|---|---|---|

|
||
| according to 1I7H | ||
| Mass / length primary structure | 111 amino acids | |
| Cofactor | (2Fe-2S) | |
| Identifier | ||
| Gene name (s) | fdx (EcoGene) | |
| External IDs | ||
Ferredoxins are iron and sulfur-containing proteins that act as electron carriers in metabolic reactions and occur in eukaryotes and anaerobic bacteria. The human ferredoxin is called adrenodoxin . Another redox protein that was isolated from chloroplasts in spinach by Tagawa and Arnon in 1962 is called "chloroplast ferredoxin". This protein plays a role in both cyclic and non-cyclic photophosphorylation in photosynthesis . In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor and reduces the coenzyme NADP + to NADPH / H + . It takes electrons from the chlorophyll excited by sunlight and transfers them to the enzyme ferredoxin-NADP (+) - reductase.
Ferredoxins are small proteins that contain iron and sulfur atoms that are arranged in an iron-sulfur cluster . Ferredoxins act as “biological capacitors ” because the iron atom can change its oxidation state (+2 or +3). They thus act as electron carriers in biological redox reactions.
Fe 2 S 2 ferredoxins
Herbal ferredoxins
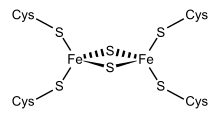
The type of ferredoxins originally found in chloroplasts are called "chloroplast ferredoxins". The active center here is a [Fe 2 S 2 ] cluster in which the iron atoms are arranged in a tetrahedral manner by inorganic sulfur atoms and by sulfur residues of the cysteine . In chloroplasts, the Fe 2 S 2 ferredoxins act as electron carriers in the electron transport chain of photosystem I and as electron donors for various proteins such as glutamate synthase , nitrate reductase and sulfur reductase . In bacterial dioxygenase systems, they serve as electron carriers between flavoprotein reductase and oxygenase .
Adrenodoxin ferredoxins
Adrenodoxin, putidaredoxin and terpredoxin are soluble Fe 2 S 2 ferredoxins that work as electron carriers . In the mitochondrial monooxygenase system, adrenodoxin transfers an electron from NADPH-adrenodoxin reductase to the membrane-bound cytochrome P450 cholesterol monooxygenase (CYP11A1) or steroid 11beta-hydroxylases (CYP11B1 or CYP11B2). The system splits off side chains and is to be found in the mitochondria of the adrenal cortex , where it contributes to the catalysis of steroid hormones . In bacteria, putidaredoxin and terpedoxin serve as electron carriers between the NADH-dependent ferredoxin reductases and soluble P450 cytochromes. Further functions of other ferredoxins of this type have not yet been clarified. Although there is no great similarity between the amino acid sequence of plant ferredoxin and adrenodoxin, both molecules have a similar folding structure.
Thioredoxin Ferredoxins
Fe 2 S 2 ferredoxin from Clostridium pasteurianum (Cp2FeFd) is recognized as a separate protein family due to its different amino acid sequence, spectroscopic properties of its iron-sulfur cluster and its unique ability to exchange ligands between cysteine and the [Fe 2 S 2 ] cluster. Although the physiological role of this ferredoxin is still unclear, a specific interaction between Cp2FeFd and the molybdenum - iron group of nitrogenase has been established. Homologous ferredoxins from Azotobacter vinelandii (Av2FeFdI) and Aquifex aeolicus (AaFd) have also been described. The crystal structure of AaFd has been clarified, AaFd is present as a dimer . The structure of the AaFd monomer differs from other Fe 2 S 2 ferredoxins. The folding of the secondary structure includes α and β folds, with the first four β chains and two α chains adopting a variant of the thioredoxin fold.
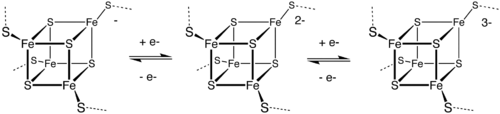
Fe 4 S 4 and Fe 3 S 4 ferredoxins
Fe 4 S 4 ferredoxins are further subdivided into "low-potential" ferredoxins (LPF) (for bacteria) and "high-potential" (HiPIP) ferredoxins. Both categories are similar in the scheme of redox reactions:
With LPF the oxidation numbers of iron can be [2Fe 3+ , 2Fe 2+ ] or [1Fe 3+ , 3Fe 2+ ], with HiPIP [3Fe 3+ , 1Fe 2+ ] or [2Fe 3+ , 2Fe 2+ ].
Bacterial ferredoxins
One type of Fe 4 S 4 ferredoxins originally found in bacteria is called a "bacterial type". Bacterial ferredoxins can in turn be divided into further subgroups, depending on the amino acid sequence present. Most contain at least one preserved domain which includes four cysteine residues that bind to the [Fe 4 S 4 ] cluster. In the ferredoxin of Pyrococcus furiosus , a preserved cysteine domain is replaced by aspartic acid .
During the evolution of bacterial ferredoxins, gene duplications and gene exchanges led to the appearance of proteins with several iron-sulfur centers. In some bacterial ferredoxins, one of the duplicated domains has lost one or more preserved cysteine residues. These domains have either lost their iron-sulfur binding property or bind to a [Fe 3 S 4 ] cluster instead of to a [Fe 4 S 4 ] cluster. The 3-D spatial structures for some bacterial ferredoxin mono- and dicluster are now known. The fold is α and β, with 2-7 α-turns and four β-strands forming a barrel- like structure and a pushed-through loop containing three proximal cysteine ligands of the iron-sulfur cluster.
High-potential iron-sulfur proteins
High-potential iron-sulfur proteins (HiPIPs) represent their own family of Fe 4 S 4 ferredoxins, which act in the anaerobic electron transport chain . Some HiPIPs have higher redox potentials than any other iron-sulfur proteins (for example, the HiPIP of Rhodopila globiformis has a redox potential of around 450 mV). The structure of some HiPIPs has since been clarified, their folds are due to α and β folds. As in other bacterial ferredoxins, the [Fe 4 S 4 ] cluster takes on a cuban- like structure and is linked to the protein by four cysteine residues.
literature
- KM Ewen, M. Kleser, R. Bernhardt: Adrenodoxin: the archetype of vertebrate-type [2Fe-2S] cluster ferredoxins. In: Biochimica et Biophysica Acta . Volume 1814, Number 1, January 2011, pp. 111-125, doi : 10.1016 / j.bbapap.2010.06.003 , PMID 20538075 .
- G. Hanke, P. Mulo: Plant type ferredoxins and ferredoxin-dependent metabolism. In: Plant, cell & environment. Volume 36, Number 6, June 2013, pp. 1071-1084, doi : 10.1111 / pce.12046 , PMID 23190083 .
- J. Meyer: Ferredoxins of the Third Kind. In: FEBS letters. Volume 509, Number 1, November 2001, pp. 1-5, PMID 11734195 .
- M. Bruschi, F. Guerlesquin: Structure, function and evolution of bacterial ferredoxins. In: FEMS microbiology reviews. Volume 4, Number 2, 1988 Apr-Jun, pp. 155-175, PMID 3078742 .
- S. Ciurli, F. Musiani: High potential iron-sulfur proteins and their role as soluble electron carriers in bacterial photosynthesis: tale of a discovery . In: Photosynth. Res. . 85, No. 1, 2005, pp. 115-131. doi : 10.1007 / s11120-004-6556-4 . PMID 15977063 .
- Fukuyama, K .: Structure and function of plant-type ferredoxins . In: Photosynth. Res. . 81, No. 3, 2004, pp. 289-301. doi : 10.1023 / B: PRES.0000036882.19322.0a . PMID 16034533 .
- Grinberg, AV, Hannemann, F., Schiffler, B., Müller, J., Heinemann, U. and Bernhardt, R .: Adrenodoxin: structure, stability, and electron transfer properties . In: Proteins . 40, No. 4, 2000, pp. 590-612. doi : 10.1002 / 1097-0134 (20000901) 40: 4 <590 :: AID-PROT50> 3.0.CO; 2-P . PMID 10899784 .
- Holden, HM, Jacobson, BL, Hurley, JK, Tollin, G., Oh, BH, Skjeldal, L., Chae, YK, Cheng, H., Xia, B. and Markley, JL: Structure-function studies of [ 2Fe-2S] ferredoxins . In: J. Bioenerg. Biomembrane . 26, No. 1, 1994, pp. 67-88. doi : 10.1007 / BF00763220 . PMID 8027024 .
Web links
- Adrenodoxin in the EBI database
- HiPIPs in the EBI database
- X-ray structure of a ferredoxin from Aquifex aeolicus
Individual evidence
- ↑ Adrenodox. In: Lexicon of Biology. Spectrum of Science , accessed September 13, 2016 .