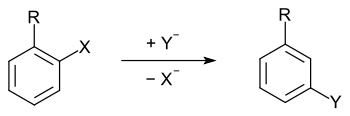cine substitution
In organic chemistry, cine substitution is a special case of substitution reactions on aromatic compounds in which the new substituent takes up a position adjacent to the leaving group on the aromatic ring.
As a rule, in nucleophilic substitution reactions, the nucleophile is bound to the same carbon atom on which the leaving group (leaving group) is located. In more general terms: The exchange of ligands takes place at the same center. However, some nucleophilic substitution reactions on benzenoid (aromatic) compounds ( nucleophilic aromatic substitution ) do not proceed according to this principle; the entering group can be bound in a different ring position than those initially occupied by the leaving group.
The US chemists Joseph F. Bunnett and Roland E. Zahler suggested the term “ cine substitution” for these cases , “from the Greek cine , to move ”. In ancient Greek κινέω (kineo) means, among other things, to move, to chase away, to flee. In German should cine So kine are spoken, analogue kinetics or cinema (Engl. Cinema !).
The term cine -substitution is a purely phenomenological description that viewed the reaction as a " single process ". In reality, however, cine substitutions consist of several sub-steps and can also take place according to different reaction mechanisms. Therefore the classification of reactions according to this term is of limited informative value.
Examples
Substitutions on halo-nitrobenzenes (nitro-halobenzenes)
Probably the oldest example of this type of reaction was described in 1871 by V. von Richter, who found that 3-bromobenzoic acid was formed when 4-bromonitrobenzene was reacted with potassium cyanide in ethanol / water ( Von-Richter reaction ).
Dow phenol synthesis
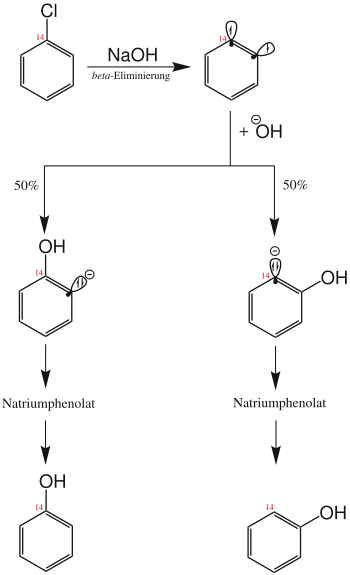
When by the US company Dow developed phenol synthesis , the reaction is of chlorobenzene with superheated aqueous sodium hydroxide solution . In today's preparative chemistry, however, it is of little importance. The observation that 50% of the product's hydroxyl group was on the C-1 atom of the benzene ring and 50% on the adjacent position can be explained by the reaction mechanism (see figure).
Chlorobenzene initially reacts by splitting off hydrogen chloride to form dehydrobenzene , which, as a strongly angular alkyne, is so reactive that it further reacts with the sodium hydroxide solution to form (2-hydroxyphenyl) sodium. This in turn is protonated to sodium phenolate , the sodium salt of the conjugate base of the target molecule phenol . The end product phenol is obtained by working up the sodium phenolate in an acidic medium.
Substitutions of non-aromatic compounds
The term was also used for the “abnormal course of the reaction” in the methanolysis of 2-bromo-2,3,4-trimethylcyclobutanone in the presence of sodium methoxide. In the first step, an enol or enolate is formed here. The bromine atom now in the allyl position dissociates as a bromide ion. The methoxy group entering is, however, in the 4-position.
This example shows that nucleophilic substitutions of allyl compounds, e.g. B. according to the S N 2 'mechanism, could be defined as cine substitutions. As mentioned above, nothing is gained in understanding the reactions.
Similar terms
Other terms describing the position of the input group in aromatic compounds are ipso and tele .
Individual evidence
- ^ Entry on cine-substitution . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01081 Version: 2.3.2.
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexicon of Chemistry , Spectrum Academic Publishing House, Heidelberg, 2001.
- ↑ Joseph F. Bunnett and Roland E. Zahler: Aromatic Nucleophilic Substitution Reactions , Chem. Rev. 49, 273-412 (1951), pp. 382-391, doi : 10.1021 / cr60153a002 .
- ↑ Reinhard Brückner : reaction mechanisms; Organic reactions - stereochemistry - modern synthetic methods , 3rd edition, Spektrum Akademischer Verlag, Munich (2004), page 254f., ISBN 9783827415790 .
- ↑ JM Conia and MJ Robson: Ring constrictions and ring enlargements of vicinally disubstituted cyclobutanes or cyclopropylmethyl compounds Angew. Chem. , 87, 505-516 (1975). doi: 10.1002 / anie.19750871404 .
literature
- Siegfried Hauptmann : Organic Chemistry. Harri Deutsch publishing house, Frankfurt a. M. 1985, 1st edition, pp. 301-302, ISBN 3-342-00280-8 .