Isoctene
Isooctene (diisobutylene, diisobutene) is a mixture of isomers from the group of unsaturated, aliphatic hydrocarbons (more precisely the alkenes ). It is isomeric to 1-octene and is a mixture of the two isomeric forms 2,4,4-trimethyl-1-pentene and 2,4,4-trimethyl-2-pentene .
Extraction and presentation
Isooctene can be obtained from isobutylene .
properties
| Isooctenes | |||||||||||||||||
| Surname | Isooctene (mixture of isomers) | 2,4,4-trimethylpentene | 2,4,4-trimethyl-1-pentene | 2,4,4-trimethyl-2-pentene | |||||||||||||
| other names |
|
|
|
||||||||||||||
| Structural formula |
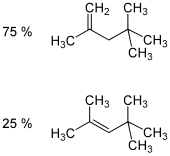
|
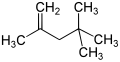
|

|
||||||||||||||
| CAS number | 11071-47-9 | 25167-70-8 | 107-39-1 | 107-40-4 | |||||||||||||
| PubChem | 7868 | 7869 | |||||||||||||||
| Molecular formula | C 8 H 16 | ||||||||||||||||
| Molar mass | 112.22 g mol −1 | ||||||||||||||||
| Physical state | liquid | ||||||||||||||||
| Brief description | volatile, highly flammable, colorless liquid with a gasoline-like odor | ||||||||||||||||
| Melting point | −50 ° C | −106 ° C | −93 ° C | −106 ° C | |||||||||||||
| boiling point | 118-125 ° C | 98-105 ° C | 101 ° C | 105 ° C | |||||||||||||
| density | 0.73 g / cm 3 | 0.72 g / cm 3 | 0.72 g / cm 3 | 0.72 g / cm 3 | |||||||||||||
| Vapor pressure | 50 mbar (38 ° C) | 103 mbar (38 ° C) | 110 mbar (38 ° C) | 111 mbar (38 ° C) | |||||||||||||
| solubility | 1.8–2.6 mg / l (20 ° C) | 26 mg / l (22 ° C) | practically insoluble | - | |||||||||||||
|
GHS labeling |
|
|
|
|
|||||||||||||
| H and P phrases | 225-304-315-411 | 225-304-411 | 225-411 | 225-304-315-319-335-411 | |||||||||||||
| no EUH phrases | no EUH phrases | no EUH phrases | no EUH phrases | ||||||||||||||
| ? |
210-261-273-301 + 310 305 + 351 + 338-331 |
210-273 |
210-261-273-301 + 310 305 + 351 + 338-331 |
||||||||||||||
use
Isooctene is used as a gas chromatographic standard, for the production of 2,4,4-trimethyl-1-pentanol and 3,3-dimethylbutene and as a starting material for the oxo synthesis of nonanols . These in turn are used as base materials for the production of plasticizers or surfactants .
Derivatives
- Diisobutylene oxide C 8 H 16 O, CAS number: 63919-00-6
safety instructions
Isooctene vapors form an explosive mixture with air ( flash point 7 ° C)
Individual evidence
- ↑ Patentscope: Diisobutylene process
- ↑ a b c d e f Entry on isooctene in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ a b c d e f Entry on 2,4,4-trimethylpentene, mixture of isomers in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ a b c d e f Entry on 2,4,4-trimethyl-1-pentene in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ↑ a b c d e f Entry on 2,4,4-trimethyl-2-pentene in the GESTIS substance database of the IFA , accessed on October 14, 2012(JavaScript required) .
- ^ Christoph Janiak, Hans-Jürgen Meyer, Dietrich Gudat, Ralf Alsfasser: Riedel Modern Inorganic Chemistry . 4th edition. Walter de Guyter., Berlin / Boston, ISBN 978-3-11-024900-2 , pp. 822 .



