Potassium benzoate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
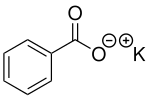
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium benzoate | ||||||||||||||||||
| other names |
E 212 |
||||||||||||||||||
| Molecular formula | C 7 H 5 KO 2 | ||||||||||||||||||
| Brief description |
white to off-white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 160.21 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
> 300 ° C |
||||||||||||||||||
| solubility |
approx. 492 g / l (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium is the potassium - salt of benzoic acid . It is approved as a food additive E 212 .
effect
Potassium benzoate has a bacteriostatic and fungistatic effect and is therefore used as a food additive (E 212) to preserve food (optimum effect at pH <3.6). This means that the treated food remains preserved over a longer period of time. Potassium benzoate is only suitable for preserving sour or sour products. It protects against mold , yeast , unwanted secondary fermentation and, in combination with sulfur dioxide, also against bacterial spoilage. It is less effective against lactic acid bacteria and against several pathogens.
use
Potassium benzoate can be synthesized in a Henkel reaction by thermal- catalytic disproportionation with the addition of carbon dioxide , potassium terephthalate :
As a by-product produced benzene .
Alternatively, potassium benzoate can be used as a preservative in the following foods: mayonnaise, marinades, ready-made salads, cake fillings, margarine, fruit juices, liquid tea concentrates, jellies, jams, vegetables pickled in vinegar, oil or brine, fish products, unheated dairy products, chewing gum, mustard, Condiments, diet foods and flavored mineral waters.
It is also used as a lubricant in the manufacture of tablets and as a corrosion inhibitor for water-bearing systems.
compatibility
Potassium benzoate can cause allergies and hives . People with asthma , hay fever or skin allergies are particularly susceptible to this additive. Especially allergy sufferers who are sensitive to acetylsalicylic acid should avoid potassium benzoate. Combinations with the preservatives sulfur dioxide (E 220), sodium sulfite (E 221), sodium hydrogen sulfite (E 222), sodium disulfite (E 223), potassium disulfite (E 224), potassium sulfite ( E 225 ), calcium sulfite ( E 226 ), calcium hydrogen sulfite ( E 227 ) and with azo dyes increase the negative effects.
Individual evidence
- ↑ a b Data sheet potassium benzoate from AlfaAesar, accessed on December 15, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Entry on potassium benzoate in the GESTIS substance database of the IFA , accessed on December 16, 2019 (JavaScript required)
- ↑ a b Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set , John Wiley & Sons, Hoboken, New Jersey, 2009 , ISBN 978-0-471-70450-8 , p. 1380.

