Potassium peroxomonosulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium peroxomonosulfate | |||||||||||||||
| other names |
|
|||||||||||||||
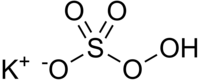
| Molecular formula | KHSO 5 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 152.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.1-1.2 g cm -3 |
|||||||||||||||
| Melting point |
90 ° C (decomposition) |
|||||||||||||||
| solubility |
easily soluble in water (256 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium peroxomonosulfate is an inorganic chemical compound of potassium from the group of sulfates and the potassium salt of peroxomonosulfuric acid .
Extraction and presentation
Potassium peroxomonosulfate can be obtained by reacting potassium carbonate or potassium hydroxide with peroxomonosulfuric acid .
properties
Potassium peroxomonosulfate is a crystalline white odorless solid that is readily soluble in water. It decomposes when heated above approx. 90 ° C. Its aqueous solution reacts strongly acidic. There is also a monohydrate with a monoclinic crystal structure .
use
Potassium peroxomonosulfate is used as an oxidizing agent in chemical synthesis, as a disinfectant in swimming pools, in medicine and in water treatment, as a bleaching and cleaning additive for textiles and dentures and as a caustic agent for surface treatment. It is also used to make the wool shrink-proof. The triple salt of potassium peroxomonosulfate, potassium hydrogen sulfate and potassium sulfate 2KHSO 5 · KHSO 4 · K 2 SO 4 is also sold under the name Oxone or Caroat . This is used for the halogenation of a, b-unsaturated carbonyl compounds and for the catalytic production of hypervalent iodine compounds for alcohol oxidation and for the synthesis of oxaziridines and dioxiranes such as dimethyldioxirane . This is the basis of Shi epoxidation .
Individual evidence
- ↑ a b c d e f g h Entry on pentapotassium bis (peroxymonosulfate) bis (sulfate) in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 978-0-412-30120-9 , pp. 3535 ( limited preview in Google Book search).
- ^ Albert Matlack: Introduction to Green Chemistry, Second Edition . CRC Press, 2010, ISBN 978-1-4398-8211-5 , pp. 86 ( limited preview in Google Book search).
- ↑ Johann Mutschmann, Fritz Stimmelmayr: Paperback water supply . Springer-Verlag, 2013, ISBN 978-3-663-12397-2 , pp. 191 ( limited preview in Google Book search).
- ↑ HELM AG: Kaliumperoxomonosulfat , accessed: July 15, 2016.
- ↑ Entry on potassium hydrogen peroxomonosulfate. In: Römpp Online . Georg Thieme Verlag, accessed on April 19, 2017.
- ↑ F.von Rheinbaben, MH Wolff: Handbook of virus effective disinfection . Springer-Verlag, 2013, ISBN 978-3-642-56394-2 , pp. 127 ( limited preview in Google Book search).
- ↑ Data sheet Oxone, monopersulfate at AlfaAesar, accessed on July 15, 2016 ( PDF )(JavaScript required) .
- ↑ Michael Frohn, Yian Shi: Chiral Ketone-Catalyzed Asymmetric Epoxidation of Olefins. In: Synthesis. 2000, p. 1979, doi : 10.1055 / s-2000-8715 .



