Enantioselective Catalysis
The enantioselective catalysis or asymmetric catalysis is concerned with the preparation of enantiomerically pure chemical compounds from achiral starting materials through a chemical reaction. For this purpose, a chiral (and enantionmerenreine) compound is used as catalyst employed.
principle
The principle of enantioselective catalysis is based on the use of chiral catalysts, mostly transition metal complexes with chiral ligands and prochiral substrates. The chirality of the products is determined by the stability of the transition state (catalyst-substrate complex) that is formed with this complex. If the enantiotopic half-spaces of the catalyst and educt fit together ( matched pair ), then the transition state is energetically lower than if the half-spaces do not fit together sterically ( mismatched pair ). The two possible transition states therefore have different stabilities and the resulting different product formation speeds. As a result, one enantiomer is preferentially formed. The enantioselectivity can be indicated by the enantiomeric excess ( enantiomeric excess or ee value) or the enantiomeric ratio ( er value).
Chiral Ligands and Catalysts
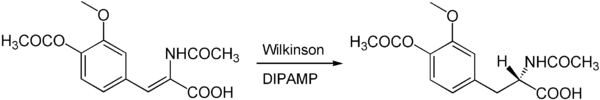
Since chirality cannot be created out of nothing, a chiral auxiliary (chiral auxiliary) must be involved in every enantioselective reaction . Since catalysts are always used in small amounts (0.1–20 mol%) in homogeneous catalysis and a catalyst can go through many cycles, it generates many product molecules with a defined conformation. The chirality of the ligands of the catalyst often comes from chiral molecules that occur enantiomerically pure in nature and can be isolated from plants or animals, the so-called chiral pool . An example is the amino acid L- proline . More often, however, specially artificially designed ligands are used. An example is the enantioselective hydrogenation with the chirally modified Wilkinson catalyst :
Enantiotopic half-spaces
- If a molecule has an axis, then it has two homotopic half-spaces , which means that a racemate is formed when an achiral group is attached to the molecule if no chiral auxiliary is involved.
- If a prochiral molecule belongs to the point group , then it has two enantiotopic half-spaces , i.e. that is, two enantiomers are formed when an achiral group is added
- Molecules that belong to the point group form diastereomers when a chiral group is attached ; one speaks here of diastereotopic half-spaces
Industrial application
The Monsanto process for the production of (L) -DOPA laid the foundation for industrial enantioselective catalysis in 1974. In terms of quantity, the largest applications are the synthesis of metolachlor and the Takasago process for the production of menthol . Other enantioselective processes are Sharpless epoxidation ( glycidol , disparlure ) and enantioselective cyclopropanation for the preparation of cilastatin and pyrethroids .
Examples
In the past few decades several Nobel Prizes in Chemistry have been awarded for enantioselective catalysis.
Oxidations
- Jacobsen epoxidation
- Sharpless epoxidation of allylic alcohols ( Nobel Prize (2001): Barry Sharpless )
Reductions
- Enantioselective hydrogenation with BINAP (Nobel Prize (2001): Ryoji Noyori )
- Enantioselective hydrogenation (Nobel Prize (2001): William S. Knowles )
- Reduction with BINAL-H
Individual evidence
- ↑ Bernd Schäfer: Menthol . In: Chemistry in Our Time . tape 47 , no. 3 , 2013, p. 174-182 , doi : 10.1002 / ciuz.201300599 (here p. 178).