Metolachlor
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Mixture of four stereoisomers (enantiomers and atropisomers), see stereochemistry | ||||||||||
| General | ||||||||||
| Surname | Metolachlor | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 15 H 22 ClNO 2 | |||||||||
| Brief description |
|
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 283.80 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
1.12 g cm −3 |
|||||||||
| Melting point |
−62.1 ° C |
|||||||||
| boiling point |
100 ° C (0.0538 hPa ) |
|||||||||
| Vapor pressure |
<0.1 Pa (20 ° C) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Metolachlor is a mixture of four isomeric chemical compounds from the group of carboxamides and chloroacetanilides .
history
Metolachlor was developed by Ciba-Geigy . In the 1970s the effect of ( rac ) -metolachlor was observed and a synthetic method and a patent were applied for. From 1978 the compound was produced on a large scale (> 10,000 t per year) and from 1982 the effect of the stereoisomers was investigated individually, whereby the particular effectiveness of ( S ) -metolachlor was found. After several attempts with catalysts made from rhodium and iridium compounds , a process with an iridium- Josiphos complex (iridiumferrocenyldiphosphine) was developed for large-scale production in 1993. In the same year the patent for ( rac ) -metolachlor expired and in 1996 the large-scale production of ( S ) -metolachlor started. Today this is one of the most frequently used herbicides in the USA. In Germany, Austria and Switzerland ( RS ) -metolachlor is not contained in any approved pesticides. In contrast, ( S ) -metolachlor is contained in several plant protection products in many EU countries and in Switzerland. Accordingly, the groundwater in Switzerland is polluted with metolachlor and the various metabolites of metolachlor.
Extraction and presentation
Today, metolachlor is obtained through stereoselective synthesis. It can be obtained by reacting 6-ethyl-2-toluidine with methoxyacetone or 2-bromomethoxypropane and then reacting with chloroacetic acid chloride.
Stereochemistry
Metolachlor occurs in two enantiomeric forms ( R ) - or ( S ) -metolachlor, with the ( S ) form being the more effective. For this reason, production processes in which the ( S ) shape is increasingly produced have been preferred for some time . This form is sold as ( S ) -metolachlor with more than 80% of the ( S ) -form.
A special feature is that the two epimers are also present as atropisomers , so there are four stereoisomers of metolachlorine. Both atropisomers of ( S ) -metolachlor [(α R , 1 ' S ) - and the (α S , 1' S ) -isomer] have the same biological effect. On the other hand, both atropisomers of ( R ) -metolachlor [(α R , 1 ' R ) - and (α S , 1' R ) -isomer] are inactive.
properties
The flash point of metolachlor is 190 ° C and the ignition temperature is 435 ° C.
use
Metolachlor is used as a herbicide (often in combination with other herbicides such as terbuthylazine or atrazine ) against grasses and millet weeds in maize, soybeans, peanuts and cotton. It works by inhibiting elongases and the geranylgeranyl pyrophosphate (GGPP) cyclizing enzymes in gibberellins .
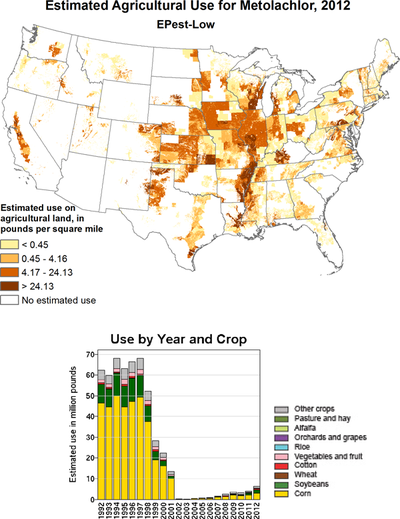
In the US, ( RS ) -metolachlor was replaced by ( S ) -metolachlor from 1998 to 2002 , of which more than 18,000 tons were used in 2012.
safety instructions
The use of metolachlor in decorative objects and games is not permitted. It is very toxic to aquatic organisms.
See also
Individual evidence
- ↑ a b c data sheet Metolachlor at Extoxnet, accessed on June 12, 2016.
- ↑ a b c d e f g h i j k l m Entry on metolachlor in the GESTIS substance database of the IFA , accessed on December 30, 2019(JavaScript required) .
- ↑ entry on S-metolachlor (ISO); 2-chloro-N- (2-ethyl-6-methylphenyl) -N - [(2S) -1-methoxypropan-2-yl] acetamide; (RaSa) -2-chloro-N- (6-ethyl-o-tolyl) -N - [(1S) -2-methoxy-1-methylethyl] acetamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 30, 2019. Manufacturers or distributors can extend the harmonized classification and labeling .
- ↑ Data sheet Metolachlor, PESTANAL at Sigma-Aldrich , accessed on December 30, 2019 ( PDF ).
- ↑ Hans-Ulrich Blaser, Elke Schmidt: Asymmetric catalysis on industrial scale: challenges, approaches and solutions. Wiley-VCH, Weinheim 2004, ISBN 3-527-30631-5 , p. 68.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on metolachlor in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 11, 2016.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on S-metolachlor in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 11, 2016.
- ↑ Plant protection products in groundwater. In: bafu.admin.ch . Retrieved November 4, 2019 .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 37 ( limited preview in Google Book search).
- ↑ H.-U. Blaser: The Chiral Switch of (S) -Metolachlor: A Personal Account of an Industrial Odyssey in Asymmetric Catalysis . In: Advanced Synthesis & Catalysis . tape 344 , no. 1 , 2002, p. 17-31 , doi : 10.1002 / 1615-4169 (200201) 344: 1 <17 :: AID-ADSC17> 3.0.CO; 2-8 .
- ↑ H.-U. Blaser: Industrial asymmetric hydrogenation "Made in Switzerland" . In: News from chemistry . tape 58 , no. 9 , 2010, p. 864-867 , doi : 10.1002 / nadc.201074031 .