Studtite
| Studtite | |
|---|---|
| Colorless to pale yellow, fibrous studtite from the Krunkelbach mine , Menzenschwand , Germany (image width: 4 mm) | |
| General and classification | |
| chemical formula | [(UO 2 ) (O 2 ) (H 2 O) 2 ] (H 2 O) 2 |
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
04.GA.15 ( 8th edition : IV / H.01) 05.03.01.01 |
| Similar minerals | Metastudtite |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic; 2 / m |
| Room group (no.) | C 2 / c (No. 15) |
| Lattice parameters |
a = 14.07 Å ; b = 6.72 Å; c = 8.43 Å β = 123.36 ° |
| Formula units | Z = 4 |
| Physical Properties | |
| Mohs hardness | soft; 1 to 2 |
| Density (g / cm 3 ) | measured: 3.58 (synthetic); calculated: 3.64 |
| Cleavage | no |
| Break ; Tenacity | pliable fragments |
| colour | yellow to light yellow; almost colorless in transmitted light |
| Line color | light yellow |
| transparency | transparent to translucent |
| shine | Glass or wax gloss |
| radioactivity | very radioactive |
| Crystal optics | |
| Refractive indices |
n α = 1.545 n β = 1.555 n γ = 1.680 |
| Birefringence | δ = 0.135 |
| Optical character | biaxial positive |
| Other properties | |
| Chemical behavior | Conversion to metastudite by dehydration |
Studtite is a very rarely occurring mineral from the mineral class of " oxides and hydroxides ". It crystallizes in the monoclinic crystal system with the chemical composition [(UO 2 ) (O 2 ) (H 2 O) 2 ] (H 2 O) 2 , so it is a water-containing uranyl peroxide . In addition to its anhydrous form metastudtite , it is the only known peroxide mineral.
Studtite only develops small, light yellow to almost colorless crystals with a needle-like crystal habit . It is mostly found in the form of fibrous mineral aggregates or crusty coatings. The transparent to translucent crystals have a glass or wax-like sheen . The mineral is generally described as soft ( Mohs hardness about 1 to 2) and the fine crystal needles are flexible.
Etymology and history
The mineral was first found in 1947 by the Belgian mineralogist Johannes Franciscus Vaes (1902–1978) in the Shinkolobwe uranium mine in Katanga (now the Democratic Republic of the Congo ). After chemical analysis, he initially thought it was a uranium carbonate, probably due to inclusions and impurities. Vaes named the new mineral after the German geologist Franz Eduard Studt , who created a geological map of Katanga in 1908. In 1974, Kurt Walenta was able to use crystallographic comparisons with known, artificially produced crystals to show that the mineral is a uranyl peroxide hydrate. It was not until 2003 that the structure of this mineral was finally elucidated by Peter C. Burns and Karrie-Ann Hughes using X-ray single crystal structure analysis.
classification
In the outdated, but partly still in use 8th edition of the mineral classification according to Strunz , the studtite belonged to the mineral class of "oxides and hydroxides" and there to the department of "uranyl ([UO 2 ] 2+ ) hydroxides and hydrates", where he together with Ianthinit , Metaschoepit , Metastudtit , Paraschoepit and Schoepit formed the unnamed group IV / H.01 .
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also classifies the Studtite in the "uranyl hydroxides" department. However, this is further subdivided according to the possible presence of further cations and, if available, also according to the crystal structure, so that the mineral can be found in the sub-section “without additional cations” according to its composition, where it is only together with metastudite the “studtite group “With the system no. 4.GA.15 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns studtite to the class of "oxides and hydroxides" and there in the department of "uranium and thorium-containing oxides". Here it can also be found together with Metastudtite in the unnamed group 05.03.01 within the sub-section " Oxides containing uranium and thorium with a cation charge of 8+ (AO 4 ), and containing water ".
Crystal structure
 Crystal structure of studtite |
|
| Crystal system | monoclinic |
| Room group (no.) | C 2 / c (No. 15) |
| Lattice parameters |
a = 14.07 Å b = 6.72 Å c = 8.43 Å β = 123.36 ° |
| Formula units | Z = 4 |
Studtite crystallizes in the monoclinic crystal system in the space group C 2 / c (space group no. 15) with the lattice parameters a = 14.07 Å (1 Å = 100 pm ), b = 6.72 Å, c = 8.43 Å and β = 123.36 ° and four formula units per unit cell .
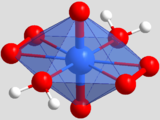
The crystal structure contains only one crystallographically distinguishable uranium atom, which is located in the origin of the unit cell (position coordinates: 0, 0, 0) and is multiplied by the existing symmetry elements to a symmetrically equivalent position. Due to its position at the origin of the unit cell, it is the only particle in the crystal structure that lies on an element of symmetry; it is located on an inversion center and has the position symmetry 1 . The uranium atom is in the form of a uranyl cation [UO 2 ] 2+ (U – O bond length : 1.77 Å), which is additionally supported by two peroxide ions O 2 2− (O – O bond length: 1.46 Å; U – O distance: 2.35 Å and 2.37 Å) and two water molecules H 2 O (U – O distance: 2.40 Å), resulting in a total coordination number of CN = 8. The resulting coordination polyhedron of the uranium atom is a distorted hexagonal bipyramid, with the oxygen atoms of the uranyl cation at the tips ( axial or apical position) and the peroxide ions and water molecules in the hexagonal base of the bipyramid ( equatorial position).
The [(UO 2 ) (O 2 ) 2 (H 2 O) 2 ] bipyramids are not isolated in the crystal structure, but rather link via the peroxide ions (i.e. via common edges) to form chains that run along the crystallographic c - Axis ([001]) and can be described with the Niggli formula : The bipyramids within the chain are inclined alternately in the opposite direction (“zigzag chain”), the chain motif repeats itself after two polyhedra or 8.43 Å, which corresponds to the lattice parameter of the crystallographic c axis.
The individual chains are linked in the crystal structure by the remaining water molecules ( crystal water ) that are not involved in the coordination sphere of the uranium atoms via hydrogen bonds , which creates the three-dimensional structure.
properties
The mineral is radioactive due to its uranium content of up to 63.6% . Taking into account the natural decay series or any decay products present, the specific activity is given as 113.9 k Bq / g (for comparison: natural potassium 0.0312 kBq / g). The quoted value can vary significantly depending on the mineral content and composition of the levels; selective enrichment or depletion of the radioactive decay products is also possible and changes the activity.
Modifications and varieties
In 1983 Michel Deliens and Paul Piret reported for the first time about the water-free form of studtite, metastudtite (UO 2 ) (O 2 ) (H 2 O) 2 . They examined several mineral samples from Shinkolobwe, and were able to prove the first natural occurrence of metastudtite by comparison with synthetically produced material. These samples are associated with rutherfordin , becquerelite , masuyite , kasolite , wölsendorfite , uranophane , soddyite and uraninite . The mineral is pale yellow and the fine fibers up to 3 mm long with a diameter of approx. 0.001 mm; it shows no fluorescence under either short-wave or long-wave UV light. The lattice parameters are given with a = 6.51 (1) Å; b = 8.78 (2) Å; c = 4.21 (1) Å and 2 formula units per unit cell specified. The type mineral is deposited in the Royal Museum for Central Africa in Tervuren , Belgium .
Education and Locations

As a secondary mineral, studtite forms very rarely in uranium deposits . Temperatures below 90 ° C and only a small amount of water, for example in the form of thin films, are required on the mineral surface. The peroxide group, known only in the minerals studtite and its water-free form metastudtite, is formed during the radiolysis of water through the alpha radiation given off by the uranium . With an average energy of 5.5 MeV, this has a range of around 40 μm in water, so that mineral formation takes place in a very local area. This produces among others the hydroxyl radical (• OH), the superoxide - radical (O 2 • - and the) hydroperoxide radical (HO 2 •), which then hydrogen peroxide (H 2 O 2 finally react), so that Form peroxide anions. In addition, reducing compounds such as hydrogen are also formed , which, however, is relatively inert chemically at temperatures below 100 ° C and can escape from the water without further chemical reactions. Since the natural radioactivity of uranium only causes relatively little radiolysis, long contact times of the uranyl ion with the radiolytically decomposed water of several hundred thousand years promote the formation of studtite. Example calculations by other authors show, however, that assuming that the hydrogen peroxide does not decompose over a longer period of time, a sufficiently high concentration for mineral formation can already have arisen after 2100 years. In principle, a sufficient amount of peroxide must be present and concentrated in the water for the formation of the mineral, which can only be achieved in thin films and long contact times.

In Shinkolobwe (Democratic Republic of the Congo) the mineral is associated with uranophane , rutherfordin and lepersonnite . Parageneses with billietite , rutherfordin, barite , quartz , hematite and limonite are known from Menzenschwand (Germany) . Finds from studtite in Tengchong (China) show paragenesis with tengchongite , calcurmolite and kivuit .
In addition to natural uranium deposits, studtite was also found in uranium-containing waste from the Hanford Site nuclear facility and in the lava-like Corium remains of the Chernobyl disaster . Although it is a very rare mineral in nature, it is considered an important aging product of radioactive waste . This is related to the formation conditions of studtite, which are seldom reached in natural uranium deposits, but can more easily arise on the surfaces of uranium-containing waste. Among other things, the mineral has been proven to be a major aging product on fuel element casings in fountain ponds. This mineral formation could also be verified synthetically in deionized water with uranium (IV) oxide (UO 2 ), which was doped with α-emitters or irradiated from external sources. The interaction of spent nuclear fuel with groundwater can thus - in addition to the formation of studtite - also lead to the formation of secondary uranium minerals such as the uranyl oxide hydrate schoepit . Since uranyl minerals can reduce the mobility of other radionuclides through incorporation into the crystal lattice and through the formation of inclusion compounds, they are important factors when considering long-term effects with regard to the solubility behavior of radioactive waste and spent nuclear fuel. Studtite is therefore important for nuclear disposal .
In addition to the type locality in Shinkolobwe, Studtit was also found in the Swambo Mine and in the Lusungu River District in Sud-Kivu . Studtit is known in Germany from the Krunkelbach mine near Menzenschwand , Wittichen and Oberwolfach , among others . In Austria , Studtit was found in Mühlbach am Hochkönig and St. Johann im Pongau . Other locations are Linópolis in Brazil , Yingjiang and Tengchong in China , Mariánské Lázně and Javorník in the Czech Republic , Lodève , Davignac and several places in the Deux-Sèvres department in France and Krøderen in Norway .
Precautions
Due to the strong radioactivity of the mineral, mineral samples from Studtite should only be kept in dust- and radiation-tight containers, but especially never in living rooms, bedrooms or workrooms. Likewise, because of the high toxicity and radioactivity of uranyl compounds, absorption into the body ( incorporation , ingestion ) should be prevented in any case and, for safety, direct body contact should be avoided and face masks and gloves should be worn when handling the mineral.
See also
literature
- JF Vaes: Six nouveaux minéraux d'urane provenant de Shinkolobwe (Katanga). In: Annales de la Société Géologique de Belgique. 1947, pp. B212-B226 ( online (French, PDF, 441 kB); on naming p. 2, mineral description p. 12).
- Studtite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( online (PDF 66.9 kB)).
- Metastudtite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( online (PDF 69.1 kB)).
Web links
Individual evidence
- ↑ a b c d e f g h i Peter C. Burns, Karrie-Ann Hughes: Studtite, [(UO 2 ) (O 2 ) (H 2 O) 2 ] (H 2 O) 2 : The first structure of a peroxide mineral. In: American Mineralogist. 2003, 88, pp. 1165-1168 ( PDF 217.8 kB ).
- ↑ a b c d Studtite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF 66.9 kB )
- ↑ a b c d e Studtite at Webmineral.com .
- ↑ a b c d Mindat - Studtite .
- ^ Paul F. Kerr: Cattierite and vaesite: New Co-Ni minerals from the Belgian Congo. In: American Mineralogist , 30, 1945, pp. 483–497 ( PDF 949.8 kB ).
- ↑ Vaesite , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF 62.3 kB ).
- ^ VDC Daltry: The type mineralogy of Africa: Zaire , In: Annales de la Société Géologique de Belgique. T. 115 (fascicule 1) 1992, pp. 33-62 ( PDF 2.2 MB ; p. 3).
- ↑ congoforum.be: Les Mineraux de la RDC (French).
- ↑ Robert Halleux, Geert Vanpaemel, Jan Vander Missen, Andrée Despy-Meyer: Geschiedenis van de wetenschappen in Belgium. 1815-2000. Dexia, Brussels 2001, Volume 2, p. 252 (Dutch, online at dbnl.org , or both volumes as PDF 48.4 MB ).
- ^ Franz Eduard Studt; Jules Cornet; Henri Jean François Buttgenbach: Carte géologique du Katanga et notes descriptives. Impr. Veuve Monnom, Brussels 1908 ( detailed description at hathitrust.org ).
- ↑ a b Kurt Walenta: On studtite and its composition. In: American Mineralogist. 1974, 59, pp. 166-171 ( PDF 646.2 kB ).
- ↑ Michel Deliens, Paul Piret: Metastudtite, UO 4 • 2H 2 O, a new mineral from Shinkolobwe, Shaba, Zaire. In: American Mineralogist. 1983, 68, pp. 456-458 ( PDF 313 kB ).
- ↑ a b G. Sattonnay, C. Ardois, C. Corbel, JF Lucchini, M.-F. Barthe, F. Garrido, D. Gosset: Alpha-radiolysis effects on UO 2 alteration in water. In: Journal of Nuclear Materials. 2001, 288, pp. 11-19. doi : 10.1016 / S0022-3115 (00) 00714-5
- ↑ a b Karrie-Ann Hughes Kubatko, Katheryn B. Helean, Alexandra Navrotsky, Peter C. Burns: Stability of Peroxide-Containing Uranyl Minerals. In: Science. 302, 2003, pp. 1191-1193 doi : 10.1126 / science.1090259
- ^ Peter C. Burns, Rodney C. Ewing, Alexandra Navrotsky: Nuclear Fuel in a Reactor Accident. In: Science. 335, 2012, pp. 1184–1188 doi : 10.1126 / science.1211285

![{\ displaystyle \ mathrm {{} _ {\ infty} ^ {1} \ lbrace [UO_ {2/1} ^ {t} (O_ {2}) _ {2/2} ^ {k} (H_ {2 } O) _ {2/1} ^ {t}]} \ rbrace}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7e2ab712c75495b4dedff4408db608c0f95f5c73)